IJCRR - 7(19), October, 2015
Pages: 06-10
Date of Publication: 10-Oct-2015
Print Article
Download XML Download PDF
EFFECT OF GROWTH AGE PERIOD ON BIOCHEMICAL COMPOSITION OF PLANTAGO MAJOR PLANT
Author: Lana Yousif Mutalib
Category: Healthcare
Abstract:Objectives: The present study was aimed to find the effect of growth age period of Plantago major leaves on the biochemical composition of the plant. Methods: Leaves of P. major have been assessed for it is total phenolic, total flavonoid and total tannin contents at different growth age period (vegetative and generative). Results: revealed significant differences in biochemical compositions between two growth age periods of the plant, generative leaves exhibited significant amount of flavonoid (0.12\?0.002 mg QE\g DW) and tannin contents (0.12\?0.002 mg GAE\g DW) while a significant total phenolic contents were expressed by vegetative leaves (0.015\?0.001 mg GAE\ g DW). Conclusions: The research findings emphasized great effect of growth age period on biochemical composition of the P. major leaves.
Keywords: Vegetative period, Generative period, Total phenolic content, Total flavonoid, Total tannin content
Full Text:
INTRODUCTION
Mankind has always been screened for agents to treat diseases since aliments were as old as life itself. Disease eradication has been performed by the usage of herbal remedies and medicinal plants. Everyday there were discovering of new medicinal plants. There collection must be at right season and specific growth stage for obtaining an optimized quantity of bioactive constituents [1]. Extracted phytochemical compounds from plant source are phenols, alkaloids, tannin, saponin, flavonoids and lignin which exert biological activity either as prophylactic or treating agents of various diseases such as diabetes, cancer, heart diseases and high blood pressure [2]. Recently, phytochemicals made a valuable venue of research in medical and food industry to emphasize their biological activities [3]. Phytochemical contents of plant affected by various factors. These factors comprised environmental conditions, season, plant age, growth factors and leaf maturity. The biological activity of medicinal plants changes with the respect to the plant age. Moreover, the right authenticated plant part at specified age period should be harvested in selected season before introducing the plant for the drug manufacturing process, to optimize the herbal preparation potency[4-7]. Plantago major L. is perennial herb belong to Plantaginaceae family, grows about 15 cm in height with variant size. The leaves were in rosettes having elliptical to ovate shape. The flowers are brownish-green color, small size appear on long non-ramified spikes [9]. P. major commonly known as a weed only while traditional medicine identified it is value as a medicinal plant. The plant mostly known for its therapeutic activity in wound healing properties [9,10]. Traditionally plant attributed in a number of disease curing processes distributed in worldwide like, infectious diseases, problems concerning the digestive organs, reproduction, against tumours, pain relieving, fever reducing, skin diseases, respiratory organs and the circulation [11-15]. The plant contains a number of medicinal active constituents such as phenolic compounds, flavonoids, alkaloids, irodiod glycoside, carbohydrate, lipid, vitamins and coumarin [12-22]. The present study was aimed to find the effect of different age growth period of Plantago major plant grown naturally. in Erbil city on the concentration of phytochemical more specifically total phenolic, total flavonoid and total tannin content in ethanolic extract of plant.
MATERIAL AND METHODS
Plant material collection: Leaves of Plantago major plant have been harvested at two different growth age period vegetative and generative growth periods, authenticated in Pharmacognosy Department, Pharmacy College\Hawler Medical University. Plant parts dried in shade, kept in close container at 25 0 C.
Assessment of Biochemical composition:
Plant parts have been assessed for it is biochemical composition by estimation of total phenolic, total flavonoid and total tannin contents.
Estimation of total phenol content:
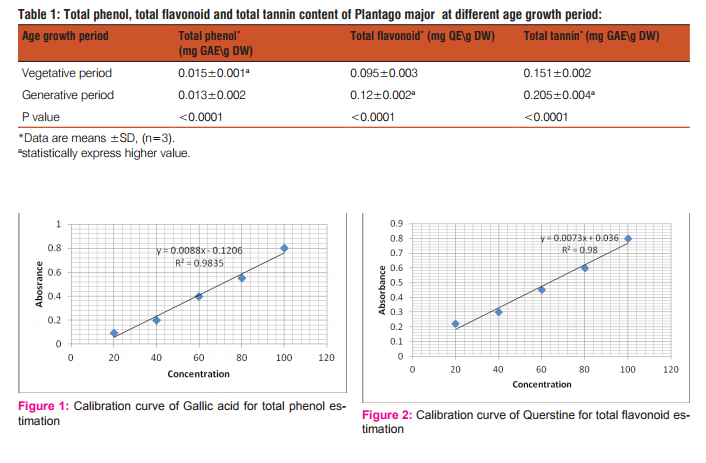
Total phenol compounds have been estimated according to the Folin-Ciocalteu method with slight modifications [23]. Briefly, 1ml extract prepared from (0.5g) of crude plant material was mixed with 9 ml of distilled water. One ml of Folin-Ciocalteu phenol reagent was added to the mixture. The mixture mixed and allowed to stand for 5 minute at room temperature, then 10ml of (7%) sodium carbonate were added. The volume have been adjusted to 25ml and incubated for 90 minutes at room temperature. The absorbance was measured at 750nm using UV visible spectrophotometer. Total phenol content were estimated from calibration curve obtained from measuring the absorbance of standard concentration of gallic acid solution in distilled water with concentrations [20, 40, 60, 80 and 100 mcg\ml]. The results were expressed as mg of gallic acid equivalent (GAE)\gram of dry powdered plant material (DW).
Estimation of total flavonoid content:
Total flavonoid content was measured by the aluminium chloride colorimetric method [24]. Aliquot of 1 ml extract prepared from 1g of powdered plant material, was added to 10 ml volumetric flask containing 4 ml of distilled water. About 0.3 ml sodium carbonate 5% was added to the flask and after 5 min, 0.3 ml aluminium chloride (10%) was added. Two ml sodium hydroxide (1 M) was added at 6th min and the total volume was adjusted up to 10 ml with distilled water. The solution was mixed thoroughly and the absorbance level was determined at 510 nm using UV visible spectrophotometer. The total flavonoid content was measured from calibration curve obtained from measuring absorbance of standard concentration of querstine solution in ethanol (80%) with concentrations [20, 40, 60, 80 and 100 mcg\ml] The results was expressed as mg of querstine equivalents (QE)\ gram plant dry weight material (DW).
Estimation of total flavonoid content:
Total flavonoid content was measured by the aluminium chloride colorimetric method [24]. Aliquot of 1 ml extract prepared from 1g of powdered plant material, was added to 10 ml volumetric flask containing 4 ml of distilled water. About 0.3 ml sodium carbonate 5% was added to the flask and after 5 min, 0.3 ml aluminium chloride (10%) was added. Two ml sodium hydroxide (1 M) was added at 6th min and the total volume was adjusted up to 10 ml with distilled water. The solution was mixed thoroughly and the absorbance level was determined at 510 nm using UV visible spectrophotometer. The total flavonoid content was measured from calibration curve obtained from measuring absorbance of standard concentration of querstine solution in ethanol (80%) with concentrations [20, 40, 60, 80 and 100 mcg\ml] The results was expressed as mg of querstine equivalents (QE)\ gram plant dry weight material (DW).
Statistical analysis: All data were collected from triplicate procedure works expressed as mean ± standard deviation (SD). Two way ANOVA method used for comparison between means considering (p value < 0.0001) statistically significant.
RESULTS
Plantago major plant have been evaluated at two different growth age period (generative and vegetative) periods for it biochemical composition using standard curves of gallic acid for total phenolic, querstine for total flavonoid and gallic acid for total tannin contents (figure.1, figure.2 and figure.3). Significant total phenolic detected in vegetative growth age period while significant total flavonoid and total tannin contents were detected in generative growth age period (p value < 0.0001) (Table.1).
DISCUSSION
Plantago major leaves is a medicinal plant used by local communities in treatment of variant diseases grown naturally in different places of Erbil city, have been assessed for their biochemical composition at different growth age periods (vegetative and generative periods), since age growth period affect phytochemical concentration in plant [4-7]. The total phenolic content of Plantago major leaves were estimated from the standard curve equation (y=0.08x - 0.120, r2 = 0.9) shown in figure.1. A significant total phenolic content were exhibited by the vegetative period leaves (p < 0.0001) in comparison to the total phenol expressed by the generative period leaves [Table .1], the finding were consistent with the finding of the Achakzai et al.2009 [6], which confirmed the low levels of phenol in young leaves of Rhododendron spp., since the plant utilized the phenol for the primary metabolic process required for plant growth. A significant amount of flavonoid content [Table.1.] were estimated in generative period of P. major leaves (p < 0.0001) from standard curve equation (y=0.007x+0.036, r2 = 0.98) shown in figure .2. Our study results were compatible to the findings of Miean and Mohamed, 2001 [26] and Albach et al, 1981 [27] and in agreement to the records of Behn et al, 2011[28] who reported high flavonoid contents in generative period leaves of lettuce, while the results were in contrast to the finding of Ali, et al 2014 [29] which reported low level of flavonoid in older leaves (generative period) in correspondence to the younger ones. Similarly to the flavonoid content, tannin contents were showed upsurge with increasing of plant age. The exhibited tannin contents of plant in different age growth periods showed significant variation in tannin contents (p < 0.0001), since generative period leaves expressed higher tannin contents [Table.1.] which have been estimated from standard curve equation (y=0.001x-0.012, r2 =0.996) shown in figure .3. Plant growth age period relation with the chemical compositions of the plant have been confirmed by Farias, 2003 [30], Esmelindro et al, 2004 [31]. Generally the chemical composition varies according to the growth age period of and it is requirements for growth. P. major leaves expressed high contents of two of evaluated phytochemical constituents flavonoid and tannin contents in generative period while the total phenolic compounds showed high values at vegetative periods. Further research have been recommended to evaluate the plant from biological activity points to standardized the medicinal age growth period of the plant.
CONCLUSION
From study results we concluded that plant growth age period reflected on the biochemical composition of the medicinal plants. Plant phytochemicals concentration either increase or decrease according age growth period of plant, choosing the right period for plant harvesting is very important for the medicinal value of the herbal preparation.
ACKNOWLEDGEMENT
Research author was very gratefully thanks Pharmacognosy Department\Pharmacy College for encouraging me to perform the research. Conflict of interest: There was no any conflict of interest to be declared by the author.

References:
1. Shahid-Ud-Daula AFM, Mohammad AB. Phytochemical screening, plant growth inhibition, and antimicrobial activity studies of Xylocarpus Granatummalaysian. J Pharm Sci 2009;7(1): 9–21.
2. Okwu D. Phytochemicals, vitamins and mineral contents of two Nigerian medicinal plants. Int J Mol Med Adv Sci 2005; 1: 375- 81.
3. Ao C, Li A, Elzaawely AA, Xuan TD, Tawata S. Evaluation of antioxidant and antibacterial activities of Ficus Microcarpa L. Fil. extract. Food Control 2008; 19: 940-8.
4. Fritz RS, Hochwender CG, Lewkiewicz DA, Bothwell S, Orians CM. Seedling herbivory by slugs in a willow hybrid system: developmental changes in damage, chemical defense, and plant performance. Oecologia 2001; 129: 87-97.
5. Pasko P, Barto H, Zagrodzki P, Gorinstein S, Fo Ta M, Zachwieja Z. Anthocyanins, total polyphenols and antioxidant activity in amaranth and quinoa seeds and sprouts during their growth. Food Chem 2009; 115: 994-8.
6. Achakzai AKK, Achakzai P, Masood A, Kayani SA, Tareen RB. Response of plant parts and age on the distribution of secondary metabolites on plants found in Quetta. Pak J Bot 2009; 41: 2129-35.
7. World Health Organization. WHO Traditional Medicine Strategy 2002-2005. Genève; 2014.
9. Anne BS. The traditional uses, chemical constituents and biological activities of Plantago Major L. (A Review). J Ethnopharmacol 2000; 71 (10): 1-21.
11. Nagata KM. Hawaiian Medicinal Plants. Econ Bot 1971; 25: 245-54.
12. Zagari A. Medicinal Plants. Tehran: Iran Book; 1992. p. 969.
13. Bocek BR. Ethnobotany Of Costanoan Indians, California, based on Collections by John P. Harrington. Econ Bot 1984; 38: 241-55.
14. Guille´n MEN, Emim JAS, Souccar C, Lapa AJ. Analgesic and anti-inflammatory activities of the aqueous extract of Plantago Major L. Int J Pharmacogn 1997; 35: 99-104.
15. Gurib-Fakim A, Sewrey M, Gueho J, Dulloo E. Medical ethnobotany of some weeds of Mauritius and Rodriguez. J Ethnopharmacol 1993; 39: 175–85.
16. Ahmed ZF, Rizk AM, Hammouda FM. Phytochemical studies of Egyptian Plantago Species (Glucides). J Pharma Sci 1965; 54, 1060-2.
17. Ahmed ZF, Hammouda FM, Rizk AM, Wassel GM. Phyochemical studies of Egyptian Plantago species. Planta Medica 1968; 4: 404-10.
18. Rojas IR. Contribucion Al estudio quimico del llanten (Plantago Major L.). Anales De La Facultad De Quimicay Farmacia 1968; 20: 146-50.
19. Pailer VM, Haschke-Hofmeister E. Inhaltstoffe aus Plantago Major. Planta Medica 1969; 17: 139-45.
20. Kawashty SA, Gamal-El-Din E, Abdalla MF, Saleh NAM.. Flavonoids Of Plantago species in Egypt. Biochem Syst Ecol 1994; 22: 729-33.
21. Bianco A, Guiso M, Passacantilli P, Francesconi A. Iridoid and phenylpropanoid glycosides from new sources. J Nat Prod 1984; 47: 901-2
.22. Zennie TM, Ogzewalla CD. Ascorbic acid and Vitamin A content of edible wild plants of Ohio and Kentucky. Econ Bot 1977; 31: 76-9.
23. Velioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J Agric Food Chem 1998; 46 (10): 4113-7.
24. Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 1999; 64 (4): 555-9.
25. Vahl EX DC, Tamilselvi N, Krishnamoorthy P, Dhamotharan R, Arumugam P, Sagadevan E. Analysis of total phenols, total tannins and screening of phytocomponents in Indigofera Aspalathoides (Shivanar Vembu). J Chem Pharm Res 2012; 4(6):3259- 62.
26. Miean KH, Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agric Food Chem 2001; 49: 3106-12.
27. Albach RF, Redman GH, Cruse RR. Annual and seasonal changes in naringin concentration of ruby red grapefruit juice. J Agric Food Chem 1981; 29: 808–11.
28. Behn H, Schurr U, Ulbrich A, Noga G. Development-dependent UV-b responses in red oak leaf lettuce (Lactuca Sativa L.): Physiological mechanisms and significance for hardening. Eur. J Hortic Sci 2011; 76: 33-40.
29. Ali G, Alireza N, Hawa ZEJ, Ali B, Izham A. Changes in phytochemical synthesis, chalcone synthase activity and pharmaceutical qualities of sabah snake grass (Clinacanthus Nutans L.) in relation to plant age. Molecules 2014; 19: 17632-48.
30. Fernandes EFA, Meloni F, Borella JC, Lopes NP. Effect of fertilisation and harvest period on polar metabolites of Calendula Oficcinalis. Rev Bras Farmacogn 2013; 23: 731-5.
31. Farias MR. Evaluations of the quality of vegetable raw materials. In: With V Diether Schenkel EP, Gosmann G, Melo JCP, Mentz LA, Petrovick PR. (Orgs.). Pharmacognosy of plant to the medicinal product. 5 ed. Porto Alegre; Florianopolis, Editora UFRGS, UFSC:2003. p. 263-88.
32. Esmelindro AA, Santos JG, Mossi A, Jacques RA, Dariva C. Influence of agronomic variables on the composition of mate tea leaves (Ilex Paraguariensis) extracts obtained from CO2 extraction at 30 0 C and 175 bar. J Agric Food Chem 2004; 52 (7): 1990-5.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License