IJCRR - 7(20), October, 2015
Pages: 17-21
Date of Publication: 20-Oct-2015
Print Article
Download XML Download PDF
BIOCHEMICAL COMPOSITION AND IN-VITRO THROMBOLYTIC ACTIVITY OF MELISSA OFFICINALIS GROWING NATURALLY IN KURDISTAN REGION\IRAQ
Author: Lana Yousif Mutalib
Category: Healthcare
Abstract:The present study was aimed to evaluate different parts of Melissa officinalis plant from biochemical composition and thrombolytic activity. Methods: Leaf and stem part of Melissa officinalis growing naturally were assessed for total phenolic, total flavonoid and total tannin contents. Both parts were examined for the invitro thrombolytic activity. Results: Significant greater amount of total phenolic (27.882 \? 0.544 mcg GAE\g DW) , total flavonoid (256.708 \? 1.447 mcg QE\g DW) and total tannin contents (424.266 \? 1.026 mcg GAE\g DW) were expressed by the leaf part (p < 0.01) in comparison to the stem part. A significant thrombolytic activity were expressed by both parts in comparison to the control negative (distilled water) (p value < 0.0001) at all tested concentration rage. Greatest thrombolytic activity were detected at high concentration (1000 mcg\ml) for both parts. Generally the thrombolytic activity exhibited by the leaf part was higher than that of stem part. Conclusion: From the results of study we concluded that leaf part of Melissa officinalis was richest in phytochemical constituents with moderate thrombolytic activity.
Keywords: Melissa officinalis leaf, Melissa officinalis stem, Thrombolytic activity
Full Text:
INTRODUCTION
Hemostasis failure of circulatory system causes thrombus formation consequently blockage of circulatory vascular system leading to series complications such as acute myocardial or cerebral infarction, at times causing death. Allopathic thrombolytic agents used are altepase, streptokinase, anistreplase, urokinase and tissue plasminogen activator (TPA) which are characterized by series side effects such as bleeding, intracranial haemorrhage, severe anaphylactic shock, and lacks specificity [1,2]. Different medicinal uses have been reported for plants from epidemiological researches and studies in addition to their usage in folk medicine. Thrombolytic activity were proven for some foods experimentally, having ability of clot lysis. Significant observations were recorded for some herbs exhibiting thrombolytic effect [3]. Melissa officinalis, family Lamiaceae is perennial aromatic herb, widely used for food and cosmetics. Different medicinal uses have been reported for the plant such as antispasmodic, anti-diabetic, antioxidant, anti-inflammatory, antibacterial and antiviral [4-10]. Variant phytochemical constituents were recorded in Melissa officinalis plant such flavonoid, tannin, phenolic compounds [11,12]. Melissa leaf was the preferred part of plant as medicinal part described by pharmacopeia, often whole herb were used as herbal remedy in some places [13,14]. Lemon balm locally known as “Trinj” is commonly used herb by Kurdish community either for culinary or medicinal purposes. The present study was aimed to find the chemical composition and thrombolytic activity of different parts of Melissa officinalis growing naturally in Erbil city, Kurdistan Region\Iraq.
MATERIALS AND METHODS:
Plant collection:
Arial parts of Melissa officinalis were collected in mountain places (Shaqlawa) of Erbil city, Kurdistan Region\Iraq at March 2015, have been identified by Department of Pharmacognosy, College of Pharmacy \ Hawler Medical University. Stem and leaf parts of plant were dried in shade. Dried plant part materials were kept in closed container under 21-23 o C.
Chemical composition:
Plant materials have been evaluated for quantitative chemical compositions by estimation of total phenolic, total flavonoid and total tannin contents of both parts separately
Estimation of total phenolic contents:
Total phenolic compounds have been estimated according to the Folin-Ciocalteu method with slight modifications [15]. One ml of extract prepared from (0.5g) crude drug was mixed with (9 ml) of distilled water. One ml of FolinCiocalteu reagent was added to diluted extract and allowed to stand at room temperature for 5 minute, then (10ml) of (7%) sodium carbonate were added. The volume have been adjusted to (25ml) and incubated at room temperature for 90 minutes. The absorbance level was measured at 750nm using UV visible spectrophotometer. Total phenolic content was estimated from calibration curve obtained from measuring the absorbance of standard concentration of gallic acid [20, 40, 60, 80 and 100 mcg\ml in distilled water]. The results were expressed as mcg gallic acid equivalent (GAE) \ g of dry weight (DW).
Estimation of total flavonoid:
Total flavonoid was measured by the aluminium chloride colorimetric method [16]. Aliquot of (1 ml) of extract prepared from (1g) was added to (10 ml) volumetric flask containing (4 ml) of distilled water. Then (0.3 ml) NaNO2 5% was added to the flask, after 5 min (0.3 ml) AlCl3 solution (10%) was also added. At 6th min, (2 ml) NaOH (1 M) was added and the total volume was made up to (10 ml) with distilled water. The solution was shaken and the absorbance level was measured versus prepared reagent blank at 510 nm. The total flavonoid content was estimated from calibration curve obtained from measuring absorbance of standard concentration of querstine [20, 40, 60, 80 and 100 mcg\ml in ethanol (80%)] The results was expressed as mcg querstine equivalents (QE)\ g dry weight (DW).
Estimation of total tannin content:
Total tannin content have been estimated according to the Folin-Ciocalteu method described by Tamilselvi et al, 2012 [17] with slight modifications. (0.1 ml) of the plant material extract prepared from (0.5g) crude drug was added with (7.5 ml) of distilled water and (0.5 ml) of Folin-Ciocalteu reagent, to the mixture add (1ml) of (35%) sodium carbonate solution. The volume completed to (10ml) with distilled water. The mixture was shaken and incubated at room temperature for 30 min and absorbance was measured at 725 nm. Total tannin content were measured from calibration curve obtained from measuring absorbance of standard concentration gallic acid [20, 40, 60, 80, 100of gallic acid\ml prepared in distilled water]. Total tannin content were expressed as mcg gallic acid equivalent (GAE)\ g dry weight (DW).
In-vitro thrombolytic assay:
Plant extract preparation:
Powdered plant parts [leaf (MOL) and stem (MOS) separately] introduce for aqueous ethanolic (80%) extraction using ultrasonic assisted extractor as described by Alupuli et al, 2009 [18]. Extracts were concentrated and dried under vacuum using rotary vapour machine. The dried extracts were reconstituted using distilled water at a concentration of 10mg\ml serving as stock solution, then a serial dilution extracts were prepared with concentrations of [200, 400, 600, 800 and 1000 mcg/ml], the solutions were kept overnight. Later the insoluble material were removed through filtration, the clear solution have been evaluated for thrombolytic activity [19,20].
Streptokinase (SK) solution preparation:
Lyophilized Streptokinase vials of 1500000 I.U commercially available in pharmacies (Abbott). One vial content were reconstituted using sterile distilled water, mixed thoroughly. This suspension was used as a standard stock from which 100μL (30,000 I.U) was used for in-vitro thrombolytic activity evaluation [19,20].
Blood sampling:
Five ml blood sample were drawn from health human volunteers (n=5), without recent history of anticoagulant and contraceptive therapy [at least 7-10 days duration]. (0.5 ml) of blood sample were transferred in a sterile aseptic condition to ten previously weighed properly labelled eppendorf tubes [19,20].
Thrombolytic assay:
Thrombolytic assay were carried out according to the described method by Sweta et al, 2007 and Daginawala et al, 2006 [19, 20]. Each properly labelled filled eppendorf tube were incubated at 37 0 C for 45 min. for clot formation. After clot formation the serum was withdrawn without disturbing the clot using syringe. Each tube was weighed again to obtain the clot weight according to the following equation:
weight of clot= weight of clot filled tube - weight of empty
tube
To each eppendorf tube add (0.1ml) of each concentration of extracts of different parts of Melissa officinalis separately, further more incubate the tubes for 90 min at 37 0 C. After incubation the supernatant fluid released from the clot lysis were removed using syringe without disturbing the clot and the tubes were re weighed. Streptokinase (SK) drug and distilled water were used as control positive and control negative respectively. The deviation in the weight of clot between two periods of incubation were expressed as percentage of clot lysis according to the following equation:
% clot lysis = [weight of released clot \ weight clot before
lysis]x 100
Statistical analysis:
All experiments were carried out in triplicate, the results were expressed as mean ± standard deviation (SD). Comparison between means were performed using one tail unpaired t-test method using Graph pad prism 6 program considering p value < 0.001 statistically significant.
RESULTS
Chemical composition
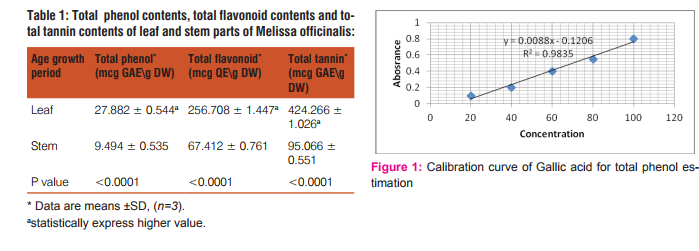
Chemical composition comprising in estimation total phenolic, total flavonoid and total tannin contents of both parts (leaf and stem) of Melissa officinalis plant from standard curves obtained from gallic acid and querstine (figure.1, figure.2 and figure.3), respectively. A significant chemical constituents have been detected in leaf part in comparison to the stem part of the plant (p value <0.0001) (Table.1.)
Thrombolytic activity:
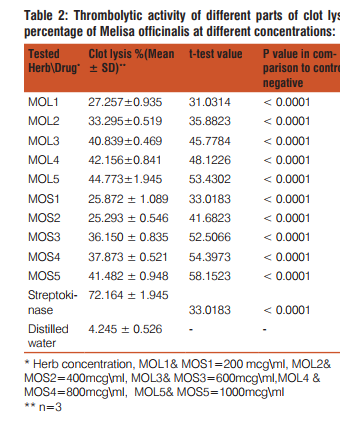
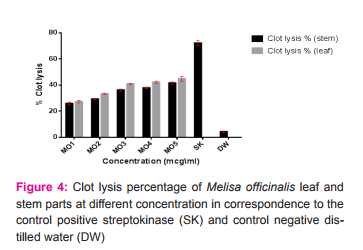
Melissa officinalis leaf and stem parts have been evaluated for thrombolytic activity, which are exhibited a linear relation-ship with concentration increment. Significant activity exhibited by both parts (p value < 0.0001) in comparison to the control negative (distilled water). Highest activity were expressed by the leaf part at concentration (1000 mcg\ml) (Table.2) and (figure.4).
DISCUSSION
Chemical composition
The chemical composition of leaf and stem parts of Melissa officinalis have been estimated inform of total phenolic, total flavonoid and total tannin contents.M. officinalis leaves exhibited a significant amount of total phenolic contents (p value <0.001) (Table.1) which have been estimated from standard curve equation of gallic acid (figure.1) in comparison to the stem part. Other biochemical compositions of the leaf part comprised in total flavonoid and total tannin contents were showed significant values (p value <0.001) in correspondence to the stem part they have been estimated from standard equation curve of querstine (figure.2) and gallic acid (figure.3), respectively. The high phytochemical contents of leaf part confirm it is activity and medicinal value of the part [13, 21].
Thrombolytic activity:
As a continuous study of cardio-protective activity of medicinal plants, Melissa officinalis plant have been assessed for it is thrombolytic activity using different plant parts as a comparison between the activity of the parts. Both parts exhibited a dose dependent manner thrombolytic activity range between (25.872 ± 1.089 - 41.482 ± 0.948%) for the leaf part and (27.257 ± 0.935 - 27.257 ± 0.935%) for the stem part, presented in Table.2. and figure .4. The thrombolytic activity expressed by both parts in comparison to the control negative (distilled water) showed significant clot lysis (p value < 0.0001) at approximately all tested concentrations after incubation for 90 minutes and moderate activity in comparison to the positive control streptokinase (30,000 IU) consequent incubation (figure.4). The greatest thrombolytic activity were detected at concentration of (1000mcg\ml) for both parts (leaf and stem). Leaf part exhibited higher thrombolytic activity in comparison to the stem part, since the concentration of phytochemicals were higher in leaf part. There were no previous works on in-vitro thrombolytic activity of M. officinalis. The activity expressed by the plant due rosmarinic acid which inhibited thromobsis in about 50% followed administration in rats [22], which was a potential active phytochemical constituent of M officinalis [23, 24].
CONCLUSION
In attempt to evaluate different parts of Melissa officinalis plant growing naturally in Erbil city, we conclude that the leaf part of plant was rich in phytochmeicals and both parts exhibit significant thrombolytic activity which may open a venue for production of new thrombolytic drugs from plant source. Further studies recommended for in-vivo thrombolytic activity of the plant.
ACKNOWLEDGEMENT
Research author was very gratefully thanks members from Ministry of Health for their cooperation for preceding the research works, and Pharmacognosy Department for encouraging me to perform the research.

References:
1. Collen D. Coronary thrombolysis: streptokinase or recombinant tissue-type plasminogen activator. Ann Intern Med 1990;112: 529–38.
2. Naderi GA, Asgary S, Jafarian A, Askari N, Behagh A, Aghdam RH. Fibrinolytic effects of ginkgo biloba extract. Exp Clin Cardiol 2005; 10(2): 85–7.
3. Yamamoto J, Yamada K, Naemura A, Yamashita T, Arai R. Testing various herbs for antithrombotic effect. Nutrition 2005; 2:580–7.
4. Enjalbert F, Bessiere J, Pellecuer J, Privat G, Doucet G. Analysis of species of Melissa. Fitoterapia 1983; 54: 59-65.
5. Auf’mkolk M, Ingbar JC, Kubota K. Extracts and auto-oxidized constituents of certain plants inhibit the receptor-binding and the biological activity of graves’ immunoglobulins. Endocrinology 1985; 116: 1687- 93.
6. Romeo V, Serena De Luca PA, Poiana M. Antimicrobial effect of some essential oils. J Essent Oil Res 2008; 20: 373-9.
7. Adjorjan B, Buchbauer G. Biological properties of essential oils: an apdated review. Flav Fragr J 2010; 25: 407-26.
8. Spiridon L, Colceru S, Anghel N, Teaca CA, Bodirlau R, Armatu, A. Antioxidant capacity and total phenolic contents of oregano (Origanum vulgare), lavender (Lavandula angustifolia) and lemon balm (Melissa officinalis) from Romania. Nat Prod Res 2011; 25:1657- 61.
9. Birdane YO, Buyukokuroglu ME, Birdane FM, Cemek M, Yavuz H. Anti-inflammatory and antinociceptive effects of Melissa Officinalis L. in rodents. Rev Méd Vét 2007; 158:75-81.
10. Chung MJ, Cho SY, Bhuiyan MJH, Kim KH, Lee SJ. Anti-diabetic effects of lemon balm (Melissa officcinalis) essential oil on glucose and lipid regulating enzymes in type 2 diabetic mice. Brit J Nutr 2010; 104: 180-8.
11. Scarpati ML, Oriente G. Isolamente e constituzione dell’acido rosmarinico (dal Rosmarinus off.). Ric Sci 1958; 28: 2329-33.
12. Adzet T, Ponz R, Wolf E, Schulte E. Content and composition of M. officinalis oil in relation to leaf position and harvest time. Planta Med 1992; 58: 562-4.
13. Pharmacopoea Bohemoslovaca, 4th ed. (PhBS IV). Prague: Avicenum; 1987.p. 101- 103/I, 409-410/III.
14. Govt. of India. Ministry of Health and Family Welfare India Pharmacopeia. New Delhi: Controller of Publications; 1996. p. A53-5.
15. Velioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J Agric Food Chem 1998; 46(10): 4113- 7.
16. Zhishen J, Mengcheng T, Jianming W. The Determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 1999; 64 (4): 555-9.
17. Tamilselvi N, Krishnamoorthy P, Dhamotharan R, Arumugam P, Sagadevan E. Analysis of total phenols, total tannins and screening of phytocomponents in Indigofera aspalathoides (Shivanar Vembu) Vahl EX DC. J Chem Pharm Res 2012; 4(6): 3259-62.
18. Alupuli A, Calinescu I, and Lavric V. Ultrasonic vs. microwave extraction intensification of active principles from medicinal plants. AIDIC Conference Series 2009; 09: 1-8
19. Sweta P, Rajpal SK, Jayant YD, Hemant JP, Girdhar MT, Hatim FD. Effect of Fagonia arabica (Dhamasa) on in vitro thrombolysis. BMC Complement Altern Med 2007; 7: 36.
20. Daginawala HF, Prasad S, Kashyap RS, Deopujari JY, Purohit HJ, Taori GM. Development of an In vitro model to study clot lysis activity of thrombolytic drugs. Thromb J 2006; 4: 14.
21.Blazek Z, Suchár A. Über die Möglichkeit der Aufnahme der Krautdrogen von Melissa officinalis L., Mentha piperita L. und Salvia officinalis L. an Stelle der Blattdrogen in die Arzneibücher. Pharmazie 1956; 11: 671-7.
22. Zou ZW, Xu LN, Tian JY. Antithrombotic and antiplatelet effects of rosmarinic acid, a water soluble component isolated from radix Salviae miltiorrhizae (danshen). Yao Hsueh Hsueh Pao 1993; 28: 241-5.
23. Anonymous. Monographs on the medicinal uses of plants. Exeter: European Scientific Cooperative on Phytotherapy; 1996.
24. Agata I, Kusakabe H, Hatano T, Nishibe S, Okuda T. Melitric acids A and B, new trimeric caffeic acid derivatives from Melissa officinalis. Chem Pharm Bull 1993; 41:1608-11.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License