IJCRR - 13(17), September, 2021
Pages: 186-191
Date of Publication: 12-Sep-2021
Print Article
Download XML Download PDF
Vegetable Preservatives (Essential Oils) of Guava (Psidium guajava L), an Alternative for Use in the Food Industry
Author: Proano-BJ, Racines-OM, Moncayo MP, Urresta VP, Vasquez CW
Category: Healthcare
Abstract:Introduction: The food industry uses synthetic preservatives in order to extend the shelf life of products and avoid their deterioration. However, some of these preservatives can affect people's health. In order to counteract this, industries are replacing them with those of vegetable origin. Guava is one option, because of its bioactive compounds. Objective: The purpose of this study was to substitute synthetic preservatives with natural compounds to offer consumers a high-quality product that guarantees their health. Method: This study evaluated the effect of seven concentrations of guava essential oils and ascorbic acid on the microbial activity (Staphylococcus aureus, Escherichia coli and Salmonella) of Frankfurter-style chicken sausages over a period of 30 days. Therefore, finding a viable solution to reduce the contamination levels of Frankfurter-style chicken sausages and to propose a natural alternative as preservative in these meat products. The collected data was analysed by means of ANOVA. Results: Of the results obtained, the application of 1000 ppm of guava essential oil alone or in combination with 700 ppm of ascorbic acid was best at inhibiting the presence of these microorganisms in the sausages, compared with the synthetic preservative (BHT). Conclusion: It is important to mention that the number of microorganisms present in the sausages were within the ranges stipulated by Ecuadorian regulations. Therefore, the obtained results demonstrated the potential of guava essential oil (Psidium guajava L) in the processed food industry, constituting a viable alternative to replace those of artificial origin.
Keywords: Antimicrobial, Antioxidant, Chicken sausage, Guava essential oil, Natural preservatives, Ascorbic acid
Full Text:
Introduction
The food industry tends to create products with synthetic preservatives to prevent food from deteriorating, extend shelf life, inhibit the growth of microorganisms and prevent the alteration of physical-chemical and organoleptic properties.1Studies related to the intake of food items containing synthetic preservatives reveal that consumers may develop health problems, such as cancer, age-related issues, vascular diseases and degenerative diseases. This is because free radicals accumulate in the body. For this reason, the global food industry is encouraging the consumption of healthy, safe and high-quality products through the use of natural preservatives of vegetable origin,2 for instance vitamins and essential oils extracted from plants. Essential oils are considered secondary metabolites that have antimicrobial, antiparasitic, insecticide, antiviral, antifungal and antioxidant properties.3,4
Guava is a tropical fruit that is distributed throughout the Americas. In Ecuador, it is even found in the Andean valleys.5Guava essential oil is obtained from the leaves, which contain bioactive compounds, such as phenolic acids, flavonoids and carotenoids, which provide greater antioxidant capacity.6,7,8 The antioxidant effect of essential oils is less than that of vitamins, especially vitamin C.9,10,11Thus, vitamin C, when combined with essential oils, enhances their properties due to a synergistic effect. In food, vitamin C acts by eliminating free radicals. It slows down the chain reactions that occur upon contact with oxygen and therefore prevents food spoilage.12,13
Given that there are bacteria, moulds and yeasts that deteriorate food, it is necessary to inhibit microbial activity with natural compounds, which have the ability to halt their multiplication.6,7,8 In this context, the antioxidant effect of guava essential oil in combination with ascorbic acid in chicken sausages was tested. There is evidence that some plant compounds have anti-microbial action, since they decelerate the growth of microorganisms in processed foods. Hence, a viable technological alternative is to replace synthetic preservatives with natural compounds to offer consumers a high-quality product that guarantees their health.
Materials and Methods
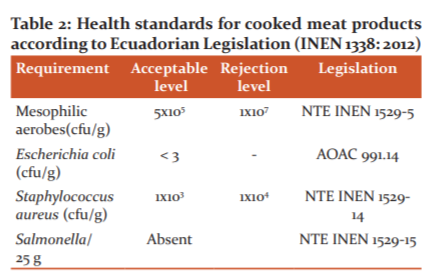
The sausages were prepared under strict hygienic practices at the Food Processing facilities of UDLA (Universidad de las Américas) using this formulation: chicken meat (40 %), chicken fat (15 %), soy protein (13 %), corn starch (7 %), salt (3 %), spices (3.5 %), sugar (2 %), carrageenan (1 %), iced water (15 %) and the proposed antimicrobial growth agents. The obtained emulsion was packed in the appropriate casings and a heat treatment was applied by immersion in hot water (82 °C until a 73 °C internal temperature was achieved) with a subsequent bath in cold water to cool the sausages down before packaging. A total of 882 chicken sausages were prepared with the desired concentrations of the proposed microbial growth inhibitors, divided into 3 repetitions that were carried out 1 month from each other. Each sausage weighed 10 g and was individually vacuum-packed for further storage. In order to contrast the antimicrobial effect of the ascorbic acid (AA) and guava essential oil (GEO), BHT (butylated hydroxytoluene) was considered as a control. The sausages were stored at a cold temperature (4 °C) and the microbial growth in the sausages was assessed every 5 days over a period of a month (days 0, 5, 10, 15, 20, 25 and 30). Seven treatments were performed, as shown below in Table 1.
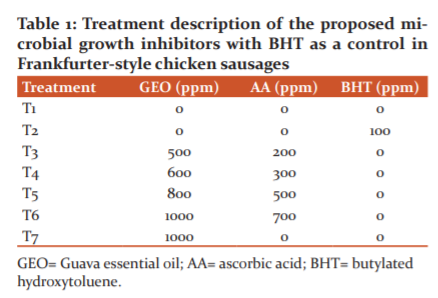
According to the Ecuadorian Food Legislation14 described in Table 2, a meat product must comply with health standards in order to be released by the industry. In this table, the method used to assess the variables is mentioned. In our study, the variables for microbial growth were: Mesophilic aerobes count (cfu/g), Escherichia coli presence (cfu/g), Staphylococcus aureus count (cfu/g) and Salmonella presence (cfu/25g).
From each sausage, 1 g was randomly obtained for the microbiological and chemical analyses. These samples were cultivated in specific media to check for microbial presence. The mesophilic aerobes assessment was performed by obtaining as stated 1 g samples and each sample was introduced into 9 ml of peptone water. An aliquot of the solution was inoculated in PCA (plate count agar) and further incubated at 37 °C for 48 h. All whitish-yellowish round colonies were counted in the tripartite petri dishes. Another sausage was used for the following analysis: 1 g sample + 9 ml of peptone water was used for Staphylococcus aureus count. This solution was inoculated into Mannitol Nutrient Agar tripartite petri dishes and further incubated at 37 °C for 48 h. All yellowish round colonies were counted. As for the E. coli assessment, another sausage was used to obtain the 1g random sample (immersed into 9 ml of peptone water) and the solution was further inoculated into Eosin Blue Metilated Agar and incubated at 37 °C for 48 h. Metallic green-colored bacteria with black-blueish centers were taken into account if present in the agar.
Meanwhile, for Samonella, SS Agar (Salmonella-Shigella Agar) was used. It was necessary to carry out a pre-enriched step with peptone water and then an enriched step with Muller Kauffman tetrathionate broth and later with Rappaport-Vassiliadis Soya broth. Finally, 100 microliters of this enriched broth that already contained the sample was placed on the SS agar at 37 ºC for 24 hours. Tripartite petri dishes were inoculated and all pink and bright red bacteria present were counted.
The cfu were counted and the following formula was used:
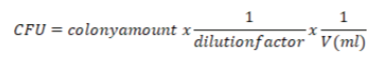
In order to assess the chemical properties of the sausages, the variables taken into account were pH and peroxide level. pH determination was accomplished using a Fished Scientific Accume potentiometer in each of the samples taken. This value was compared to the Ecuadorian Food Legislation standard that recommends a maximum of 6.2. For the peroxide level in the samples, an MQuant Peroxide semi-quantitative Test (Merck) was used with a peroxide level range of 0; 5; 2; 5; 10; and 25 mg/l H2O2.
Results
Mesophilic aerobes
When analyzing the growth data of mesophilic aerobes in the products, it was determined that there are significant differences between treatments starting at day 15 (Table 3).
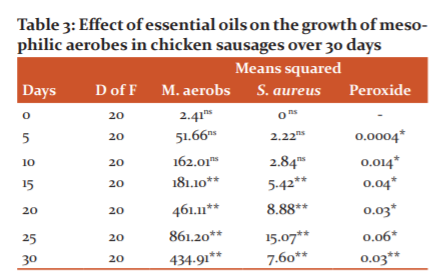
ns: no statistical differences; * Statistical differences (5%); ** Statistical differences (1%).
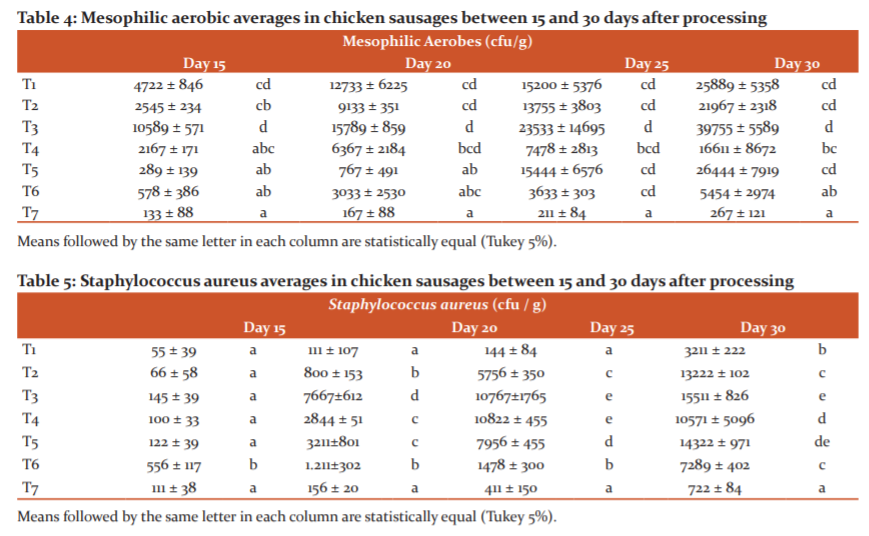
Mesophilic aerobes appeared from day 5 onwards in the product. However, from day 15, they increased consistently up to 30 days in all treatments (Table 4). Treatment T7, which contained 1000 ppm of guava essential oil, was the best at inhibiting the growth of this type of microorganism, since it presented the lowest amount of cfu / g of mesophilic aerobes (133 cfu/g), followed by T5 with more than 3 times the amount of the pathogen (767 cfu), and also T6 (1000 ppm of guava essential oil + 700 ppm of ascorbic acid) with 3033 cfu / g. The same trend occurred for the evaluations carried out at 20, 25 and 30 days (Table 4). For its part, the control (T2) containing the synthetic preservative BHT exceeded the amount recommended by Ecuadorian regulations, increasing from 9133 to 21967 cfu/g between days 15 and 30.
Means followed by the same letter in each column are statistically equal (Tukey 5%).
Staphylococcus aureus
The treatments under study affected the growth of Staphylococcus aureus as well as the aforementioned microorganism from day 15 onwards (Table 3). The amount of cfu of Staphylococcus aureus increased as the storage time of the products increased for all treatments (Table 5). Only T7 had less than 1000 cfu of Staphylococcus aureus, which complies with the provisions of the INEN standard. However, T6 did not exceed that amount by much, hence treatments 6 and 7 did not have significant statistical differences (letter c).
Escherichia coli and Salmonella
In the 30 days of investigation, neither E.coli nor Salmonella cfu grew
Peroxides
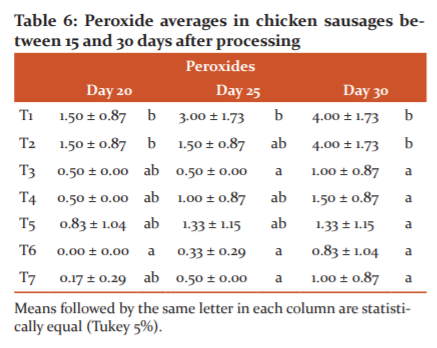
Regarding peroxides, the effect of the treatments was determined on the evaluated product during the entire evaluated period (Table 3). The average and standard deviation of the peroxide data at days 20, 25 and 30 can be seen in Table 6. From day 20, differences were identified between treatments
Discussion
Mesophilic aerobes
It is important to note that the amount of this microorganism present in the product during the time evaluated in T7 is within the parameters stipulated by Ecuadorian regulations,14which indicate that the amount must be equal to or less than 5 x 105 cfu / g for sausages, therefore we can indicate that they are suitable for human consumption.
It is important to indicate that food can contain bacteria (mesophilic aerobes), molds and yeasts, and depending on the amount of these microorganisms present, it can be evaluated whether or not a product is suitable for human consumption1.In this study, it can be indicated that the T7 sausages are safe for consumption during the 30 days of evaluation. Furthermore, this indicates that the processing and storage processes were adequate.The inhibition of mesophilic aerobes in chicken sausages with antimicrobial emulsions, which contained the essential oil of guava monoterpenes, 1,8 cineol,a- terpenil and p- cimen, among others, delayed the growth of microorganisms14,15and therefore increased the product’s shelf life.16
Treatments T6 presented the lowest averages of cfu / g of mesophilic aerobes over the 30 days. This coincides with the study by17, who determined in an in vitro study that the minimum inhibitory amount of guava essential oil in chicken sausages should be 800 ppm. The presence of ascorbic acid in T6 also helped to control the proliferation of bacteria.13
In our investigation, it was determined that all the treatments were within the parameters of the INEN Standard for sausages as regards mesophilic aerobes. In chicken sausages, the antimicrobial solutions did not totally inhibit mesophilic aerobes; however, the decrease was due to the guava essential oil in combination with ascorbic acid.
Staphylococcus aureus
The growth of Staphylococcus aureus in processed foods limits their useful life. The presence of this bacterium is considered an indicator of sanitary control1, because it can produce toxic infections in the consumer caused by thermostable toxins.1In chicken sausages, the proliferation of bacteria took place from day 0 to day 30, exceeding the admissible limits set by the INEN standard (1,000 cfu / g). The only treatment that maintained the product’s useful life until day 30 was T7. This is corroborated by the studies published by,18,19who reported that extracts of Psidium guava perform antimicrobial activity on gram-positive bacteria such as Staphylococcus aureus, determining that the minimum inhibitory concentration is between 500 and 1,000 ppm of guava essential oil. Another research group20 also found that guava extract is antibacterial due to its constituents and that it limits the growth of Staphylococcus aureus. Additionally21,22confirmed that the active compounds of guava essential oil as thermenoids provide an antimicrobial effect and inhibit some Staphylococcus aureus strains.23,24,25
The T2 treatment, which contains BHT (chemical preservative), inhibits the presence of S. aureus up to day 20. Therefore, the T7, which contains only guava essential oil, had the best results.8Rakmaia26 demonstrated that the antimicrobial activity of guava essential oil with a minimum inhibitory amount of 500 μg / ml controlled the growth of S. aureus thanks to the presence of monoterpenes such as limonene.
Escherichia coli and Salmonella
The presence of bacteria such as Escherichia coli and Salmonella spp. indicates that a product is contaminated and is unsuitable for human consumption. The Frankfurter-style chicken sausages with antimicrobial emulsions did not present colony-forming units of Escherichia coli and Salmonella sp during the thirty days of investigation. All the treatments inhibited the growth of Escherichia coli. This was due to the presence of components like flavonoids in guava,26,27 which causes the denaturation of the membrane and inhibits the proliferation of Escherichia coli.28Furthermore, studies from Bermudez et al.3confirmed the effectiveness of guava essential oil against E. coli in meat and bone meal previously contaminated with this bacterium.
Studies by Dhiman et al.8also revealed that a minimal inhibitory concentration (0.78 μg / ml of the P. guajava extract) exhibited the bacterial activity of E. coli. During the thirty days of research, there was no contamination by Salmonella spp. in the chicken sausages: all treatments inhibited this bacterium. This is consistent with the in vitro investigation of guava essential oil through the disc diffusion method, the result of which was inhibition of 9 mm.29
Arima et al.31 identified two new flavonoid glycosides, morin-3- O- α- L- lixopyranoside and morin-3- O- α- L-arabopyranoside, which with a minimum inhibition concentration of 200 μg / ml were able to inhibit Salmonella bacteria. Furthermore, guava essential oil’s anti-bacteriostatic activity against Salmonella is attributed to the presence of flavonoids.27
However, there are studies in which the effect of guava essential oil on the inhibition of gram-negative bacteria, such as Escherichia coli could not be verified. One group of investigators extracted oil from Psidium guava through various processes and demonstrated that there was no inhibition of Escherichia coli due to the permeable structure of this bacterium’s membranes, which are composed of an external lipopolysaccharide that restricts the entry of the essential oil extract.20
In addition, the inhibition of these bacteria was attributable to the effect of the scalding temperature in the formulation of the sausages, as confirmed by,30 who established that in the elaboration of sausages it is necessary to use scalding or a heat treatment. This process reduces the number of microorganisms present in the samples because it reaches a temperature of 70-75 ºC, guaranteeing the safety of the food with respect to these bacteria. In this case, the sausage production was controlled by scalding, reaching an internal temperature of 70 ºC and a water temperature of 75 °C, guaranteeing the safety of the food with respect to these bacteria.By not presenting colony-forming units of Escherichia coli and Salmonella spp. over the 30-day period, the chicken sausages were within the parameters of the INEN Standard,14 which establishes that they must be less than 3 cfu/g for Escherichia coli and that the colony-forming units must be completely absent for Salmonella spp.
Peroxides
Peroxides have an oxidizing capacity in food. They are compounds that produce lipid rancidity, which causes food to deteriorate and shortens its useful life.31From day 20 to day 30, there were significant changes between treatments, because peroxides formed due to a lipolysis process (Table 3). However, only treatments 1 and 2 exceeded the limits established by,31which suggest that a peroxide index of 10 meq / L or above in meat products causes oxidative rancidity and deterioration.
Treatments 3 to 7 controlled lipid rancidity, which agrees with,32 who determined that the presence of solutions with essential oils in meat products preserves the product by postponing the oxidation that causes its deterioration.
Conclusion
The studied treatments over the 30 days had different effects on the control of the microorganisms evaluated in the chicken sausages. The use of guava essential oil alone and in combination with ascorbic acid were the treatments that best controlled those microorganisms, having an even better effect than the synthetic preservative used as a control. In addition, they had the best action against the product's peroxides, obtaining the lowest averages.
Acknowledgements
The author acknowledges the immense help received from the scholars whose articles are cited and included in references to this manuscript. The author is also grateful to authors/editors/publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
Conflict of Interest: Nil
Source of Funding: Nil
Authors’ Contributions:
Janeth Proaño-Bastidas: Conceptualization of the research, methodology and writing- original draft preparation, review and editing.
Wilson Vasquez-Castillo: Data curation and statistical analysis. Writing-review and editing.
Mauricio Racines-Oliva: Data curation and statistical analysis. Writing-review and editing.
Pablo Moncayo Moncayo: Data validation and project administration.
Paula Urresta Valencia: Laboratory work.
References:
-
AndinoF, Castillo Y. Food microbiology: Practical approach to food safety.Estelí Nicaragua:National University of Engineering; 2010.
-
Inouye S, Takizawa T, Yamaguchi H. Antibacterial activity of essential oils and their major constituents against respiratory tract pathogens by gaseous contact. Antimicrob Chemother. 2001 May; 47 (5): 565-573.
-
Sacchetti G, Maietti S, Muzzoli MV, Scaglianti M, Manfredini S. Comparative evaluation of 11 essential oils of different origin as functional antioxidants: antiradicals and antimicrobials in food. Food Chem. 2005 August; 91: 621-632.
-
Bermúdez-Vásquez MJ, Granados-Chinchilla F, Molina A. Chemical composition and antimicrobial activity of the essential oil of Psidium guajava and Cymbopogon citratus. Agron. Mesoam. 2019 January; 30 (1): 147-163.
-
Abdelrahim SI, Almagboul AZ, Omer MEA, Elegami A. Antimicrobial activity of Psidium guajava L. Phytother Res. 2002 December;73 (7-8): 713-715.
-
Ogunwande IA, Olawore NO, Deleke KA, Ekundayo O, Koenig WA. Chemical composition of the volatile oil from Psidium guajava L. leaves growing in Nigeria. Flavour Fragr J. 2003 March 03; 18: 136-138.
-
Chen HC, Sheu MJ, Lin LY, Wu CM. Chemical composition of the essential oil of Psidium guajava L. leaf from Taiwan. J. Essent. Oil Res. 2011 December 28;19 (4): 345-347.
-
Metwally AM, Omar AA, Ghazy NM, Harraz FM, Sohafy SM. Monograph of Psidium guajava L. leaves. Pharmacogn. J. 2011 April; 3, (21): 89-104.
-
Dhiman A, Nanda A, Ahmad S, Narasimhan B. In vitro antimicrobial activity of methanolic leaf extract of Psidium guajava L.J Pharm Bioallied Sci.2011 June 03; 3(2): 226.
-
Yaaser MR, Rashad OB. Evaluation of the antioxidant and antibacterial properties in two types of Yemeni guava cultivars. Biocatal. Agric. Biotechnol. 2018 October; 16: 90-97.
-
Carvalho AV, Lima LCD. Quality of kiwis minimally processed and subjected to treatment with ascorbic acid, citric acid and calcium chloride. Pesqui. Agropecu. Bras2002; 37(5): 679-685.
-
Sangha O, Stucki G. Vitamin E in therapy of rheumatic diseases. Z Rheumatol 1998 July 31; 57 (4): 207-214.
-
Núñez A. Antioxidant therapy, oxidative stress and antioxidant products: challenges and opportunities. Cuban Rev Public Health. 2011; 37 (suppl.): 644-60.
-
INEN 1338. INEN Standard Raw, Cured, Precooked Meat Products, Requirements Quito: Ecuadorian Institute for Regularization, 2012.
-
Hsin-ChunC, Ming-JenS, Li-YunL, Chung-MayW. Chemical composition of the essential oil of Psidium guajava L. leaf from Taiwan. J. Essent. Oil Res.2011 November 18; 19:345-347.
-
Gutierrez RM, Mitchell SR. Solís R. Psidium guajava: A review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol 2008 April 17; 117 (1): 1-27.
-
De Souza L, DaméLF, Hörnke Alves G, Ziemann MA, AlvesMR.Evaluation of the bactericidal activity of essential oils of guava, pitango and arazá leaves. Rev. Cuba. de Plan Medi.2011;16(4), 324-330.
-
INEN 1529-14. Microbiological control of food Staphylococcus aureus, Quito,1998.
-
Sanches, NR, Garcia Cortez DA, Schiavini MS, Nakamura CV, Dias Filho BP. An evaluation of antibacterial activities of Psidium guajava (L.). Braz Arch. 2005 May 01;48(3): 429-436.
-
Malaviya A, Mishra N. Antimicrobial activity of tropical fruits. Bri Int J. 2011; 3 (1): 1-4.
-
Gonçalves FA, Andrade Neto M, Bezerra A, Macrae JN, Sousa OVD. Fonteles-Filho, AA and Vieira, RH et al. GUAVA antibacterial activity, Psidium guajava Linnaeus, leaf extracts on enteric bacteria causing diarrhea isolated from Seabob shrimp, Xiphopenaeuskroyeri (Heller), Rev Inst Med Trop São Paulo.2008 February; 50 (1):11-15.
-
Barahona BA. Evaluation of the in vitro antibacterial activity of two extracts of medicinal plants oregano (Lippia graveolens hbk) and guava (Psidium guajava), on Escherichia coli; causing colibacillosis in domestic birds (Gallus gallus) (Doctoral dissertation, Universidad de San Carlos de Guatemala. 2016
-
Mahfuzul MD, Bari ML, Inatsu Y, Juneja VK, Kawamoto S. Antibacterial activity of extracts of guava (Psidium guajava L.) and neem (Azadirachta indica A. Juss.) Against foodborne pathogens and spoilage bacteria. Foodborne Pathog Dis.2007 November 27; 4 (4):481-488.
-
Soliman FM, Fathy MM, Salama MM, Sabre FR.Comparative study of the volatile oil content and antimicrobial activity of Psidium guajava L. and Psidium cattleianum Sabine leaves. Boletín de la Facultad de Farmacia, Universidad de El Cairo. 2016 December; 54 (2): 219-225.
-
Martínez MJ, Molina N, Boucourt E. Evaluation of the antimicrobial activity of Psidium guajava L. (Guayaba). Rev. Cuba. de Plantas Medicinales 1997 April; 2 (1):12-14.
-
Rakmaia J, Cheirsilpa B, Mejutob J, Simal-Gándarac CJ. Torrado-Agrasarc A. Antioxidant and antimicrobial properties of encapsulated guava leaf oil in hydroxypropyl-beta-cyclodextrin. Ind Crop Prod. 2018 January; 111: 219-225.
-
Daniel VA, Moreno JE, Hidalgo DC, Martinez JRV, Borges-Argaez R, Farfan MC et al. Antimicrobial activity and chemical composition of the essential oils ofMalvaviscusarboreuscav, Pimenta dioica (L.) merr., Byrsonimacrassifolia (L.) Kunth and Psidium guajava l, Trop. Subtrop. Agroecosyst.2013;16 (3).
-
Rattanachaikunsopon P, Phumkhachorn P. Contents and antibacterial activity of flavonoids extracted from Psidium guajava plants, Rev. Cuba. de Plantas Medic. 2010; 4(5): 393-396.
-
Emmanuel A, Kubmarawa D, Sara GY, Wahu A. Phytochemical detection, antioxidant and antimicrobial activities of essential oils and ethanol extract of Psidium guajava leaf. Asian J. Phys. Chem. Sci. Ess. 2019 1-8,
-
Arima H, Danno GL. Isolation of antimicrobial compounds from guayaba (Psidium guajava L.) and its structural dilution, Biosci. Biotechnol. Biochem. 2002; 66 (8):1727-1730.
-
CODEX STAN 210. Norma del CODEX para grasas y aceites no regulados por normas individuales1999 ; 1-14.
-
AlarcónJ, Panez R, Romos P, Valle E, Yon A. Determination of the Index of Peroxides in Oils and Fats. Oil and fat technology. Federico Villareal National Univers. 2019.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License