IJCRR - 7(21), November, 2015
Pages: 19-24
Date of Publication: 11-Nov-2015
Print Article
Download XML Download PDF
PHYSIOTHERAPY FOR INTERMITTENT CLAUDICATION: A REVIEW ARTICLE
Author: Preeti S. Christian
Category: Healthcare
Abstract:Peripheral arterial disease (PAD) mainly occurs due to atherosclerotic stenosis or occlusion of the arteries of the lower limbs, resulting in an impairment of blood flow to the legs. Patients with PAD have a significant reduction in their physical activities like walking due to intermittent claudication. Intermittent claudication is a major symptom of Peripheral arterial disease. It is cramping pain, aggravated by exercise and relieved by rest. It is because of atherosclerosis, fatty deposits blocking blood flow through the arteries, which reduce blood flow to the muscles of leg. Treatments include stopping smoking, starting to physiotherapy, drugs
and surgery. This review of article found that physiotherapy can relieve intermittent claudication for many people. Exercise may be better than angioplasty. Some other types of surgeries are available which are more effective than exercise, but they carry more risks. Nowadays various modes of physiotherapy are available. It is advisable to start physiotherapy treatment with proper guidance.
Keywords: Intermittent claudication, Peripheral arterial disease, Atherosclerosis, Physiotherapy.
Full Text:
INTRODUCTION
Intermittent claudication is a symptom that describes muscle pain (ache, cramp, numbness or sense of fatigue), classically in the calf muscle, which occurs during exercise, such as walking, and is relieved by a short period of rest. The pain occurs again when the same amount of exercise is taken. It is classically associated with early-stage peripheral artery disease, and can progress to critical limb ischemia unless treated or risk factors are modified. Peripheral arterial disease (PAD) is characterised by atherosclerotic stenosis or occlusion of the arteries of the lower limbs, resulting in an impairment of blood flow to the legs. Claudication derives from the Latin verb claudicare, “to limp”.1 it is thought that 10% of patients with IC progress to critical limb ischemia and 2% require amputation.2 There are multiple classifications for which to grade the severity of claudication, such as the Fontaine scale: 4
• Stage 1 - No symptoms
• Stage 2 - Intermittent claudication 2a - no resting pain, onset of claudication in more than 200 meters 2b - no resting pain, onset of claudication in less than 200 meters
• Stage 3 - Nocturnal and/or resting pain
Stage 4 - Necrosis (death of tissue) and/or gangrene in the limb Investigation can be done by Ankle brachial pressure index, Exercise tests, Electrocardiography, Angiography.3
TREATMENT AVAILABLE FOR INTERMITTENT CLAUDICATION 3
1. MEDICAL TREATMENT:
• Medications to help control high blood pressure and cholesterol. Other drugs that may help include antiplatelet medications to prevent blood clots.
• In severe cases, procedures may be needed to open blocked blood vessels.
2. PHYSIOTHERAPY TREATMENT:
• Regular exercise, which is essential for patients with mild-to-moderate PAD.
3. OTHER MEASURES:
• Smoking cessation.
PHYSIOTHERAPY GUIDELINES:
Following are the guidelines for the management of patients with lower extremity peripheral arterial disease with complain of IC which is given by American Heart Association and American College of Cardiology (AHA/ACC): 4
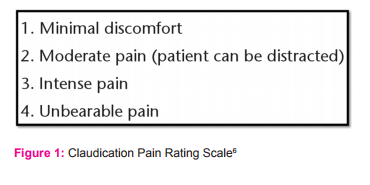
• Supervised treadmill or track walking at an intensity that elicits claudication symptoms within 3 to 5 minutes (a score of 1 on the Claudication Pain Rating Scale-Figure 1).4
• Walking until the claudication pain is rated as moderate (a score of 2 on the Claudication Pain Rating Scale- Figure 1), followed by standing or sitting rest to permit symptoms to resolve.4
• Repeating these exercise and rest cycles for 35 minutes of intermittent walking.4
• Increasing the exercise program by 5 minutes per session to 50 minutes, 3 to 5 times per week, for a minimum of 12 weeks.4
DIFFERENT MODES OF EXERCISES:
1. SUPERVISED VS NON SUPERVISED 6, 7
In regular care, exercise therapy is usually prescribed in the form of advice to “go home and walk”, without supervision or follow-up. 6 There is no evidence to support the efficacy of this advice and compliance is known to be low.7 Factors, such as fear of pain, inadequate knowledge and poor general condition, contribute to the difficulty of starting, sustaining and maintaining exercise therapy. Supervised exercise therapy (SET) entails adequate coaching to increase the maximal walking distance. Patients can be gradually transitioned to independent, unsupervised exercise over time if independent exercise is deemed safe by the program staff. At the completion of the supervised training program, patients should be given a home exercise prescription to maintain activity levels because it is expected that exercise training should be continued as a lifelong activity.6, 7
2. LOW VS HIGH INTENSITY 6, 7
Intensity can be guided by an exercise tolerance test with the use of heart rate reserve or oxygen uptake reserve.
3. WEIGHT BEARING VS NON WEIGHT BEARING 47
Weight bearing exercises: treadmill, stepper Non weight bearing exercises: cycling, rowing 4.
UPPER BODY VS LOWER BODY EXERCIS 47
Upper body exercises: Biceps curl, Triceps extension, Overhead press, Lateral raises, Bench press, Lateral pull-down/pull-ups, Bent -over/ seated row Lower body exercises: Leg extensions, curls, press, Adductor/abductor, ankle planter/dorsiflexion, toe flexion/extension
MECHANISM OF EFFECTS OF EXERCISE:
Possible mechanisms, through which exercise may mediate an improvement in intermittent claudication, are described below.
1. Increase Collateral Circulation:
Functional limitation in PAD traditionally has been ascribed to diminished blood flow induced by arterial obstruction from atherosclerotic stenosis. Typical intermittent claudication could theoretically be attributed to ischemia induced by an oxygen demand and supply imbalance. Certainly, fixed atherosclerotic lesions reflected in a diminished ABI are the precipitating event that leads to functional abnormalities in PAD. 8-11 Theoretically, enhanced distal blood flow due to vascular adaptations could underlie the benefits of exercise therapy in PAD. In animal models of arterial insufficiency, available evidence indicates that exercise training augments peripheral arterial supply.8-11 Recent studies demonstrate that exercise stimulates gains in collateral blood flow after femoral occlusion in rodent models through collateral enlargement.8, 12, 13 Collateral growth induced by exercise reflects vascular structural remodelling, a process that depends on both growth factor activity and increased nitric oxide bioavailability via shear stress stimulation of endothelial nitric oxide synthase.8,12,14
2. Improve Endothelial Health:
Normal vascular function depends on a healthy endothelium that elaborates vasoprotective factors, including nitric oxide to regulate arterial flow. Reduced nitric oxide bioavailability in the skeletal muscle microcirculation diminishes the hyperaemic flow response to ischemia and may impede augmentation of blood flow during exercise in PAD.15, 16 Two studies have demonstrated an improvement in endothelial function with exercise training in PAD. A supervised exercise program increased endothelium-dependent flow mediated dilation of the brachial artery by 65% in 19 elderly patients with intermittent claudication.17 In the randomized trial comparing treadmill exercise with lower-extremity strength training and with usual care in PAD, treadmill exercise but not lower-extremity strength training augmented flow-mediated dilation, consistent with improvement in endothelial health. McDermott and colleagues evaluated the effect of each exercise regimen on flow-mediated dilation of the brachial artery.18
3. Enhance Skeletal Muscle Metabolism and Mitochondrial Function: Metabolic dysfunction at the skeletal muscle level superimposed on compromised blood flow has the potential to magnify physical limitation. Episodic ischemia in concert with chronically low physical activity levels alters skeletal muscle phenotype in PAD patients.5 Altered skeletal muscle energetics in PAD has been linked to mitochondrial dysfunction. Abnormal mitochondrial function may interfere with skeletal muscle oxygen utilization and accelerate endothelial damage.19, 20 Decreased calf muscle area and lower type I fiber content are associated with impairments in functional performance measures.21, 22 Exercise training has the potential to enhance skeletal muscle metabolism and mitochondrial function. Interestingly, exercise-induced capillary growth in skeletal muscle also depends on peroxisome proliferators activated receptor-gamma coactivator-1α, suggesting a connection between mitochondrial function and exercise adaptations relevant to PAD.24 In PAD patients, exercise training has been shown to restore carnitine metabolism in association with improved treadmill walking.25, 23
4. Suppressing Inflammatory Activation: Chronic inflammation participates in the atherosclerotic process. Systemic markers of inflammation including C-reactive protein and soluble intercellular adhesion molecule-1 increase the risk of developing PAD.26,27 Higher levels of inflammation are associated with disease progression and with adverse cardiac and lower-extremity outcomes.28-30 Inflammation may accelerate functional impairment in PAD by favouring plaque growth and inducing skeletal muscle injury. Physical activity may have favourable effects in PAD by suppressing inflammatory activation. Extensive epidemiological data demonstrate lower inflammatory marker levels in individuals who participate in regular physical activity compared with those who are sedentary.31A 3-month exercise program ameliorated neutrophils activation after treadmill exercise in 46 PAD patients with claudication.32
DISCUSSION
The earliest suggested therapy for patients with intermittent claudication was exercise therapy. In 1898, Wilhelm Erb first described the results of a patient with intermittent claudication that was successfully treated with exercise.33 The results of the first randomised clinical trial were reported in 1966 by Larsen et al.34 In this study 7 patients were instructed to take a daily walk of at least one hour, besides their normal activities. Patients had to walk until claudication pain forced cessation of exercise and, after a period of rest until the pain disappeared, patients had to repeat the exercise. The 7 patients in the control group were given “medical treatment” in the form of lactose tablets. For the group treated with exercise, a significant increase in maximum walking time was seen, whereas the patients in the control group did not improve their walking distance. Nowadays, exercise therapy is extensively studied, and according to several guidelines the therapy of first choice for patients with complaints of intermittent claudication.35, 36, 37 Housley et al (1988) indicate that “stop smoking and keep walking” has long been the standard first line of management, despite a paucity of adequate studies showing benefits.4 The optimal training program for patients with intermittent claudication should be based on repeated walking until near-maximal pain followed by a short period of rest in a frequency of at least 3 times a week for 30 minutes during a period of at least 6 months.13
SUPERVISED VS NON SUPERVISED EXERCISES: However, the adherence of patients given an oral exercise advice is low. Co-morbidity, lack of specific advice, and lack of supervision are barriers to actually perform walking exercise.39 Supervised exercise therapy (SET) performs better in increasing walking distance compared to an oral exercise advice.38 The Trans-Atlantic Inter-Society Consensus Document on the management of PAD (TASC-II) recommends with ‘level A evidence’ that supervised exercise should be made available as part of the initial treatment for all patients with PAD.40 However, in routine clinical practice most patients only receive an oral advice to increase their walking activities, since supervised exercise programs are not universally available and implemented in daily care for patients with PAD. Supervised exercise programs are more effective than nonsupervised programs in improving treadmill walking distances in patients with IC. The evidence suggests that programs focus on walking at an intensity that elicits symptoms (score of 1 on the Claudication Pain Rating Scale- figure 1) within 3 to 5 minutes, stopping if symptoms become moderate (score of 2 on the Claudication Pain Rating Scale- figure 1), resting until symptoms have resolved, and then resuming walking. The exercise program should be for 30 to 60 minutes of exercise and rest cycles per session, 3 to 5 times per week, for a minimum of 3 months time period.41, 42 A recent Cochrane Review identified a significant improvement in walking distance in patients undergoing a supervised exercise therapy (SET) program compared with those involved in a nonsupervised program, with an increased difference in maximal walking distance of approximately 150 meter after 3 months of time period.43
LOW VS HIGH INTENSITY EXERCISE: Gardner Aw et al conducted a study to find out the effect of exercise intensity on the response to exercise rehabilitation in patients with intermittent claudication. The major finding of this investigation was that PAD patients limited by intermittent claudication who completed a low-intensity exercise program had similar improvements in physical function, peripheral circulation, and health-related quality of life as those patients who completed a high intensity exercise program. In conclusion, the efficacy of low-intensity exercise rehabilitation is similar to high intensity rehabilitation in improving markers of functional independence in PAD patients limited by intermittent claudication, provided that a few additional minutes of walking is accomplished to elicit a similar volume of exercise.44
UPPER VS LOWER EXTREMITY EXERCISE: The results of the randomized controlled trial conducted by Rena Zwierska et al suggested that both upper- and lower- limb weight-supported aerobic exercise training provide an adequate stimulus for evoking improvements in walking performance in patients with PAD. Evidence from the this study suggests that the improvement in walking performance after upper-limb training is due to a combination of central cardiovascular and/or systemic mechanisms in addition to an adaptation in exercise pain tolerance that enables patients to endure a greater intensity of claudication pain before test termination. These findings demonstrate the effectiveness of alternative aerobic exercise interventions for patients with symptomatic PAD. Arm-cranking was very well tolerated by their patient cohort and at high exercise intensities using the interval training regimen. This, and other alternative exercise training modalities such as leg-cranking, and it could be a very useful strategy for improving cardiovascular function and exercise pain tolerance in patients who have become physically inactive due to the discomfort that they encounter during walking, particularly during the early stages of a rehabilitation program45
WEIGHT BEARING VS NON WEIGHT BEARING: Sanderson B et al concluded that however all forms of activity beneficial to Cardio Vascular health and fitness; nonweight bearing was more bearable still weight bearing was better, including 1.9 minutes increased time before onset of claudication. 46
CONCLUSION
Physiotherapy is very effective for patients with intermittent claudication to improve functional capacity and reduce cardiovascular risks. Patient can start with supervised program and then can switch to non supervised home program with proper selection of frequency and intensity. Patients should be encouraged to commence exercise at a moderate intensity, and should stop and rest if claudication pain becomes severe. Walking is most commonly used exercise form by patients. Other forms of exercise like cycling, arm-cranking, strengthening of large muscles of upper/lower body may also are incorporated as tolerated by patients. So physiotherapy treatment with proper guidance is very effective to relieve intermittent claudication.
ACKNOWLEDGEMENT
I am very much grateful to my loving family members and friends for their interest in my academic excellence and also for their encouragement and support. I acknowledge the great help received from the scholars whose articles cited and included in references of this manuscript. I am also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed. I am grateful to IJCRR editorial board members and IJCRR team of reviewers who have helped to bring quality to this manuscript.
ABBREVIATIONS
PAD : Peripheral arterial disease
IC : Intermittent claudication
ABI : Ankle brachial pressure index
SET : Supervised exercise therapy
References:
1. Leng GC, Fowler B, Ernst E Leng GC, Fowler B, Ernst E. Exercise for intermittent claudication. Cochrane Database of Systematic Reviews 2000;2. Art. No: CD000990. DOI: 10.1002/14651858.CD000990.
2. Edward b jude. Intermittent claudication in the patient with diabetes. Br J Diabetes Vasc Dis 2004; 4:238–42.
3. Aspi F.Golwala. Medicine for Students. In: Cardiovascular System. 22nd ed.Mumbai: Neel Graphics; 2008. p.320
4. ACSM’s Exercise Management for Persons with Chronic Diseases and Disabilities.3rd Edition. Human Kinetics; 2009.
5. Brass EP, Hiatt WR. Acquired skeletal muscle metabolic myopathy in atherosclerotic peripheral arterial disease. Vasc Med. 2000; 5:55–59.
6. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 8th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams and Wilkins; 2010
7. American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. Champaign. IL:Human Kinetics; 2004.
8. Prior BM, Lloyd PG, Ren J, Li H, Yang HT, Laughlin MH et al. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol. 2004; 287:H2434–H2447.
9. Yang HT, Dinn RF, Terjung RL. Training increases muscle blood flow in rats with peripheral arterial insufficiency. J Appl Physiol. 1990;69: 1353–1359.
10. Yang HT, Ren J, Laughlin MH, Terjung RL. Prior exercise train-ing produces NO-dependent increases in collateral blood flow after acute arterial occlusion. Am J Physiol.2002;282:H301– H310.
11. Yang HT, Prior BM, Lloyd PG, Taylor JC, Li Z, Laughlin MH et al. Training-induced vascular adaptations to ischemic muscle. J Physiol Pharmacol. 2008;59 suppl 7:57–70.
12. Lloyd PG, Yang HT, Terjung RL. Arteriogenesis and angiogenesis in rat ischemic hindlimb: role of nitric oxide. Am J Physiol. 2001;281: H2528–H2538.
13. Yang HT, Ogilvie RW, Terjung RL. Training increases collateral dependent muscle blood flow in aged rats. Am J Physiol. 1995;268:H1174–H1180.
14. Yu J, demuinck ED, Zhuang Z, Drinane M, Kauser K, Rubanyi GM et al. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci U S A. 2005;102:10999 –11004.
15. Gordon MB, Jain R, Beckman JA, Creager MA. The contribution of Nitric oxide to exercise hyperemia in the human forearm. Vasc Med. 2002;7:163–168.
16. Meredith IT, Currie KE, Anderson TJ, Roddy MA, Ganz P, Creager MA. Postischemic vasodilation in human forearm is dependent on endothelium derived nitric oxide. Am J Physiol. 1996;270:H1435–H1440.
17. Brendle DC, Joseph LJ, Corretti MC, Gardner AW, Katzel LI. Effects of exercise rehabilitation on endothelial reactivity in older patients with peripheral arterial disease. Am J Cardiol. 2001;87:324 –329.
18. Mcdermott MM, Ades P, Guralnik JM, Dyer A, Ferrucci L, Liu K et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA. 2009;301:165– 174.
19. Bauer TA, Brass EP, Hiatt WR. Impaired muscle oxygen use at onset of exercise in peripheral arterial disease. J Vasc Surg. 2004;40:488–493.
20. Bauer TA, Brass EP, Barstow TJ, Hiatt WR. Skeletal muscle sto2 Kinetics are slowed during low work rate calf exercise in peripheral Arterial disease. Eur J Appl Physiol. 2007;100:143–151.
21. Mcdermott MM, Hoff F, Ferrucci L, Pearce WH, Guralnik JM, Tian L et al. Lower extremity Ischemia, calf skeletal muscle characteristics, and functional impairment in peripheral arterial disease. J Am Geriatr Soc. 2007;55:400–406.
22. Askew CD, Green S, Walker PJ, Kerr GK, Green AA, Williams AD et al. Skeletal muscle phenotype is associated with exercise Tolerance in patients with peripheral arterial disease. J Vasc Surg. 2005;41:802– 807.
23. Hiatt WR, Regensteiner JG, Wolfel EE, Carry MR, Brass EP. Effect of exercise training on skeletal muscle histology and metabolism in peripheral arterial disease. J Appl Physiol. 1996;81:780 –788.
24. Chinsomboon J, Ruas J, Gupta RK, Thom R, Shoag J, Rowe GC et al. The transcriptional coactivator PGC-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proc Natl Acad Sci U S A. 2009;106:21401–21406.
25. Hiatt WR, Regensteiner JG, Hargarten ME, Wolfel EE, Brass EP. Benefit of exercise conditioning for patients with peripheral arterial Disease. Circulation. 1990;81:602– 609.
26. Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic Atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine,lipoprotein and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285:2481–2485.
27. Pradhan AD, Shrivastava S, Cook NR, Rifai N, Creager MA, Ridker PM. Symptomatic peripheral arterial disease in women: nontraditional biomarkers of elevated risk. Circulation. 2008;117:823– 831.
28. Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. C-reactive protein, interleukin-6, and soluble adhesion molecules as Predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation. 2005;112:976 –983.
29. Vidula H, Tian L, Liu K, Criqui MH, Ferrucci L, Pearce WH et al. Biomarkers of inflammation and thrombosis as predictors of near-term mortality in patients with peripheral arterial disease: A cohort study. Ann Intern Med. 2008;148:85–93.
30. Beckman JA, Preis O, Ridker PM, Gerhard-Herman M. Comparison of usefulness of inflammatory markers in patients with versus without peripheral arterial disease in predicting adverse cardiovascular outcomes (myocardial infarction, stroke, and death). Am J Cardiol. 2005;96:1374–1378.
31. Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol. 2005;45:1563–1569.
32. Turton EP, Coughlin PA, Kester RC, Scott DJ. Exercise training reduces the acute inflammatory response associated with claudication. Eur J Vasc Endovasc Surg. 2002;23:309 –316.
33. Nielsen SL, Gyntelberg F, Larsen B, Lassen NA. Hospital versus home training, a clinical trial. Aktuelle Prob Angiol.1975; 30: 121–126.
34. Nielsen SL, Larsen B, Prahl M, Jensen CT, Jensen BE, Wenkens V. Hospital training compared with home training in patients with intermittent claudication. Ugeskr Laeger. 1977; 139: 2733–2736.
35. Patterson RB, Pinto B, Marcus B, Colucci A, Braun T, Roberts M. Value of a supervised exercise program for the therapy of arterial claudication. J Vasc Surg.1997; 25: 312–318.
36. Cheetham DR, Burgess L, Ellis M, Williams A, Greenhalgh RM, Davies AH. Does supervised exercise offer adjuvant benefit over exercise advice alone for the treatment of intermittent claudication? A randomised trial. Eur J Vasc Endovasc Surg.2004; 27: 17–23.
37. Regensteiner JG, Meyer TJ, Krupski WC, Cranford LS, Hiatt WR. Hospital vs home-based exercise rehabilitation for patients with peripheral arterial occlusive disease. Angiology 1997; 48: 291–300.
38. Bess Fowler, Konrad Jamrozik, Paul Norman, Yvonne Allen, Eve Wilkinson. Improving maximum walking distance in early peripheral Arterial disease: Randomised controlled trial. Australian Journal of Physiotherapy.2002;48.
39. Bendermacher BLW, Willigendael EM, Teijink JAW, Prins MH. Supervised exercise therapy versus non-supervised exercise therapy for intermittent claudication. Cochrane Database Syst Rev. 2006.
40. Walker RD, Nawaz S, Wilkonson CH, Saxton JM, Pockley AG, Woods HF. Influence of upper- and lower-limb Exercise training on cardiovascular function and walking distances in patients with intermittent claudication. J Vasc Surg. 2000; 31: 662–669.
41. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45:S5–S67.
42. Hirsch AT, Haskal ZJ, Hertzer NR,et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. J Am Coll Cardiol. 2006;47:1239–1312.
43. Bianca L Bendermacher, Edith M Willigendae, Saskia P Nicolaï,Lotte M Kruidenier, Rob J Welten, Erik Hendriks et al.Supervised exercise therapy for intermittent claudication in a community-based setting is as effective as clinic-based. J Vasc Surg. 2007 Jun;45(6):1192-6.
44. Gardner AW, Montgomery PS, Flinn WR, Katzel L. The effect of exercise intensity on the response to exercise rehabilitation in patients with intermittent claudication. J Vasc Surg. 2005 Oct;42(4):702-9.
45. Irena Zwierska, Richard D. Walker, Sohail A. Choksy, Jonathan S. Male, A. Graham Pockley, John M. Saxton. Upper- vs lowerlimb aerobic exercise rehabilitation in patients with symptomatic peripheral arterial disease: A randomized controlled trial. J Vasc Surg. 2005 Dec;42(6):1122-30.
46. Sanderson B, Askew C, Stewart I, Walker P, Gibbs H, Green S. Short-term effects of cycle and treadmill training on exercise tolerance in peripheral arterial disease. J. Vasc. Surg. 2006 Jul;44(1):119-27.
47. Naomi M. Hamburg, MD; Gary J. Balady, MD. Exercise Rehabilitation in Peripheral Artery Disease Functional Impact and Mechanisms of Benefits. Circulation. 2011; 123:87-97.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License