IJCRR - 13(16), August, 2021
Pages: 68-76
Date of Publication: 30-Aug-2021
Print Article
Download XML Download PDF
Evaluation of the Anti-Diabetic Activity of Sophora Interrupta: Pharmacological Screening Against Streptozotocin-Induced Diabetic Rats
Author: Faheem IP, Gopalakrishna B, Mohsina FP, Sarah Priya
Category: Healthcare
Abstract:Introduction: Diabetes Mellitus (DM) is a highly prevalent metabolic disorder characterized by chronic hyperglycaemia. Though multiple conventional therapies are available these therapies are reported to have side effects. Aim: To evaluation of the anti-diabetic activity of Sophora interrupta by using the streptozotocin-induced diabetic rat model. Plants are potential sources of phytoconstituents with varied pharmacological activities. Methodology: The leaves and the stem bark of Sophora interrupta(SI) were collected and initial results of the phytochemical screening revealed that all the bark and leaf extract of the plant showed the presence of flavonoids, Saponins, steroids, alkaloids, tannins, phenolic compounds, triterpenoids and carbohydrates. Results: The results of acute toxicity studies demonstrated that animals did not display any drug-related behavioural, physiological and psychological changes. The streptozotocin model was used for the induction of diabetes. In diabetic rats, decreased bodyweight, High-density lipoprotein (HDL), reduced glutathione (GSH), Superoxide dismutase (SOD), Catalase (CAT) and increased level of Blood glucose, total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL), very-low-density lipoprotein (VLDL) and TBARs was observed which was normalized by 200 mg/kg and 500 mg/kg extract of the plant. Conclusion: It shows that the ethanolic extract of SI showed significant antioxidant and antidiabetic activity directing it as a better therapeutic regimen in the treatment of diabetes and associated complications.
Keywords: Plant extract, High-density lipoprotein, Reduced glutathione, Total cholesterol, Triglycerides, Low-density lipoprotein, Very low-density lipoprotein
Full Text:
-
Introduction
DM is a metabolic disorder of multiple etiology characterized by chronic hyperglycemia with disturbances of carbohydrate, fat and protein metabolism resulting from defects in insulin secretion, insulin action, or both.1Diabetes is the sixth leading cause of death worldwide.2
Several pathophysiological processes are involved in the development of DM. These range from autoimmune destruction of the pancreatic β-cells with consequent insulin deficiency to abnormalities that result in resistance to insulin action. Deficiency and insufficient action of insulin on target tissues lead to carbohydrates, fats and proteins metabolism abnormalities.3
Oxidative stress has been suggested as a contributory factor in the pathogenesis of DM(4). Diabetes increases the production of tissue-damaging reactive oxygen species (ROS) by glucose autoxidation and/or non-enzymatic protein glycosylation (5). Hyperglycemia has been found to increase the production of ROS such as superoxide anion (O2–), and hydrogen peroxide (H2O2) which reduce nitrogen oxide (NO) bioavailability in cultured endothelial cells, and in vascular tissue (6). Endothelial dysfunction is a well-documented characteristic phenomenon in DM (5–7), and is attributed to decreased vasorelaxant, and increased contractile responses to physiological, and pharmacological stimuli.4
In conventional medical practice, the present therapies of DM are reported to have side effects. For instance; sulfonylurea causes weight gain due to hyperinsulinemia, biguanide cause body weakness, fatigue, lactic acidosis and alpha glucosidase inhibitor may cause diarrhoea while thiazolidinediones may increase LDL-cholesterol level.8
This raises the need for other sources of these inhibitors that have fewer side effects.9 Therefore, because of the side effects associated with the present antidiabetic drugs, there are need to develop effective, safe and cheap drugs for diabetes management. Such effective, safe and cheap drugs could be obtained by using medicinal plants which have been used by humans to prevent or cure diseases including diabetes since the dawn of civilization.10
Medicinal plants are used by almost 80% of the world’s population for their basic health care because of their low cost and ease in availability.11The use of herbal medicinal plants has always played a positive role in the control or prevention of diseases such as diabetes, heart disorders and various cancers.12 Some medicinal plants have been used in the production of various drugs singly or in combination and even as principal raw material for the production of other conventional medicines.13 Extracts from the other common species have also been used as medicine in treating various illnesses.14 Therefore, traditional medicine offers promising solutions to face the global increasing demands for new therapeutic agents. Insufficient data exist for most plants to guarantee their quality, efficacy and safety.15 However, the adverse effects of phytotherapeutic agents are less frequent compared with synthetic drugs, but well-controlled clinical trials have now confirmed that such effects really exist.16 In present study, pharmacognostic and pharmacological profile Sophora interrupta(SI) plant is explored.SI plant shows a plethora of pharmacological effects including its role in cancer treatment.17,19 as anti-ulcer. 20 anthelmintic.17 hepatoprotective21 and antioxidant activity. This cumulative data shows that SI plant have a wide spectrum of therapeutic potential. Thus, the beneficial effects of individual plant extracts in STZ-induced diabetes was assessed in the present study.
2. MATERIALS AND METHODS
2.1. Collection and Authentication of Sophora interrupta Plant
Fresh leaves and bark of SI were collected from Tirumala hills, Chittoor district from the state of Andhra Pradesh. The plant materials were taxonomically identified and authenticated by Dr. MadhavaChetty, Asst. Professor, Dept. of Botany, S.V. University, Tirupathi Andhra Pradesh, India and the sample voucher specimen and herbarium have been preserved in the Dept. Of Pharmacognosy, Luqman College of Pharmacy Gulbarga, Karnataka.
2.2. Preliminary phytochemical screening of SI plant extracts22,23
The phytochemical screening of leaf and bark of FD, SI and CM were carried out for the detection of alkaloids, carbohydrates, glycosides, phenolic compounds, flavonoids, protein and free amino acids, saponins, sterols, acidic compound, steroids, fixed oil and fats and terpenoids.23,24,25,26,27
2.3. Experimental animals:
Age-matched young Wistar rats weighing about 200-250 g were employed in the study. The animals have housed at approximately 24±1°C temperature and humidity of 55±5% with a 12-hour light/dark cycle. The animals were fed with a standard diet (standard chow from Ashirwad Industries, Ropar, India) and water ad libitum. The animals were acclimatized for at least 3-4 days before the initiation of the experiment and were observed for any sign of disease. The animals were maintained under proper conditions throughout the study. The experimental protocol was approved by the Institutional Animal’s Ethics Committee. The animals were sacrificed after a predetermined period of the treatment as per the study design to evaluate various parameters.
2.4. Acute toxicity study
Acute toxicity includes the effects of a single dose of a chemical/substance (or several doses within a 24-hour period) on the whole body, usually manifested over a period of 14 days. In the current study, acute toxicity of plant extracts were as per Organisation for Economic Co-operation and Development (OECD) guidelines. Acute toxicity study was performed in accordance with OECD guidelines 425.28 No adverse effect or mortality was detected in albino rats up to 3 gm/kg, per oral of extracts during the 24 to 72 hr observation periods. For this period the rats were continuously observed for 5 hr for any gross behavioral, neurological or autonomic toxic effect and lethal Fly after 24 to 72 hrs.
2.5. Induction of diabetes
Animals were injected with a single dose of streptozotocin (STZ; 65 mg/kg, i.p.) prepared in fresh citrate buffer (pH 4.5). The development of diabetes was confirmed after 72 h of the STZ injection. The animals having fasting blood glucose levels of more than 250 mg/dL were selected for the study.29
2.6. Experimental Protocol
Experimental animals were divided into five different groups (eight animals each). The plant extracts were evaluated for their antidiabetic effect at doses of 500 mg/kg per oral (p.o.) and glipizide (4 mg/kg, p.o.).
Group I (Untreated normal control rats): Normal control rats received only a normal diet and water during the experimental period but without any therapy.
Group ll (Plant extract-treated normal rats): Normal rats treated with a single dose of aqueous extract of SI orally at a dose of 500 mg/ kg daily one time, for 14 consecutive days.
Group III (Diabetic control rats): Rats of this group were STZ-induced diabetic models and were served as diabetic controls throughout the experimental period but without any therapy.
Group IV, V, VI (Plant extract treated diabetic rats): Diabetic models of rats treated with a single dose of aqueous extract of SI orally at a dose of 100, 200, 500 mg/kg daily one time, for 14 consecutive days.
Group VII (Glipizide Treated Diabetic Group): The diabetic rats after 1 week of STZ administration were treated with glipizide (4 mg/kg, p.o.) for 2 weeks.
2.7. Estimation of body weight
The body weight of each animal was measured before induction of STZ and periodically till the end of the study.
2.8. Biochemical estimation:
Blood samples were collected (under light anesthesia) by retro-orbital puncture method after overnight fasting and analyzed for Blood glucose level, lipid profile [Serum total cholesterol (TC), triglycerides (TG), low-density lipoproteins (LDL), very-low-density lipoproteins (VLDL) and high-density lipoproteins (HDL)]. Biochemical estimation was carried out using available laboratory kits of Erba Diagnostics Pvt. Ltd.
2.8.1 Estimation of serum glucose
Blood glucose level was estimated after 72 hours of STZ administration to confirm diabetes. Fasting blood glucose level was estimated on the 0th day, the 30th day and the 75th day.
2.8.2. Assessment of Blood lipid profile
The total cholesterol was estimated by cholesterol oxidase peroxidase
CHOD-POD (cholesterol oxidase-peroxidase) method (30) and serum triglyceride was estimated by glycerophosphate oxidase peroxidase GOD-POD method (31). The HDL was assayed by cholesterol oxidase peroxidase CHOD-POD method using manufacturer kit (30). Serum VLDL and LDL concentrations were calculated according to the Friedewald equation (Frideewald & wt, 1972).
LDL cholesterol = Total cholesterol (TC) – High-density lipoprotein (HDL) -Triglycerides (TG)/5.
2.8.3. Assessment of oxidative stress in serum samples
The oxidative and antioxidant parameters in serum samples were assessed by estimating TBARS (thiobarbituric acid reactive substance), GSH (glutathione), CAT (catalase) and SOD (superoxide dismutase) levels.Lipid peroxidation was determined by TBARS concentrations, which was spectrophotometrically measured at 532 nm(33). SOD and GSH-Px levels were determined using kits. SOD activity was measured by the method of Misra andFridovich(34). The GSH level was estimated using the methods described by Ellman(35). A standard curve was plotted using the reduced form of glutathione (0.1–1 mM), and the results were expressed as mM/g protein. Serum CAT activity was assayed using the Spectrophotometric method (36) at 620 nm and expressed as micromoles of hydrogen peroxide decomposed/min/milligram protein.
2.9. Statistical Analysis
Data were presented as mean± S.EM. For continuous variables, a student t-test was used to differentiate the mean difference. For comparison between more than 2 groups, the data were processed by one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test. *p < 0.05 was considered significant. Statistical analysis was performed using SPSS version 21.
3. Results
3.1 Preliminary Phytochemical screening in the leaves and barks extracts of SI Plant
Preliminary phytochemical screening of the crude, 1:10 and 1:100 extracts of all the four solvents (n-hexane, chloroform, ethanol and water) was performed in order to characterize the classes of compounds that are present in the leaf and bark of SI plant (Table 1). This qualitative screening included the tests were performed using reference methods (37,38). The results of the phytochemical screening revealed that all the bark and leaf extracts of the plant showed the presence of flavonoids, Saponins, steroids, alkaloids, tannins, phenolic compounds, triterpenoids and carbohydrates. All four extracts were found positive for alkaloids, flavonoids, phenols tannins, carbohydrates, saponins, cardiogylcoside and proteins. On the other hand, steroids and Anthraquinones are not present in either of the extracts. Moreover, ethanolic extract of leaf of SI (ELESI), aqueous extract of leaf of SI (WELSI), ethanolic extract of bark of SI (EBESI) and aqueous extract of bark of SI (WEBSI) found richer in these phytoconstituents as compared to hexane and chloroform extracts. Glycosides, anthraquinones, and reducing sugars are not present in either of the extract.
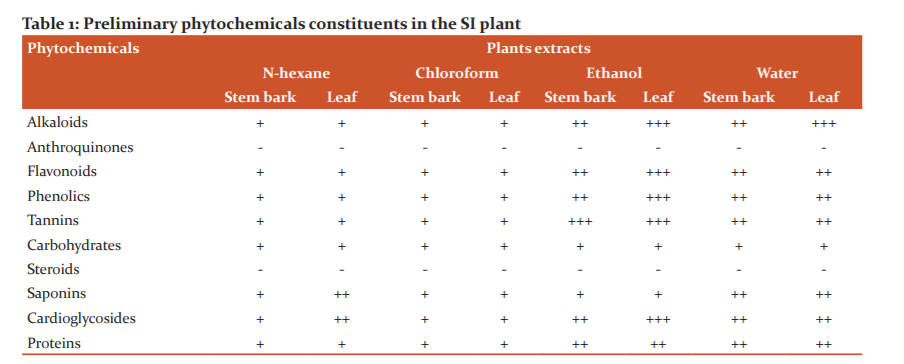
Note: “+++” indicates positive (Most Abundance), “++” indicates positive (Moderately present), “+” slightly positive, “–” completely absent.
3.2. Quantitative estimation of total phenolic, flavonoids and tannin content in the leaf and bark extracts of FD, SI and CM plants
The results for the total phenol, tannin and flavonoid estimation of all four extracts of SI are tabulated in table 2. The total phenolic, tannin and flavonoids content of n-hexane, Chloroform, ethanol and aqueous extracts in SI leaf part was ranged from 1.5 -27.5, 1.8-2.7 and 2.3-29.2 g GAE/100 g extract and in bark part was ranged from 3.2 -32.1, 2.6-3.4 and 3.1-35.3 g GAE/100 g extract respectively.
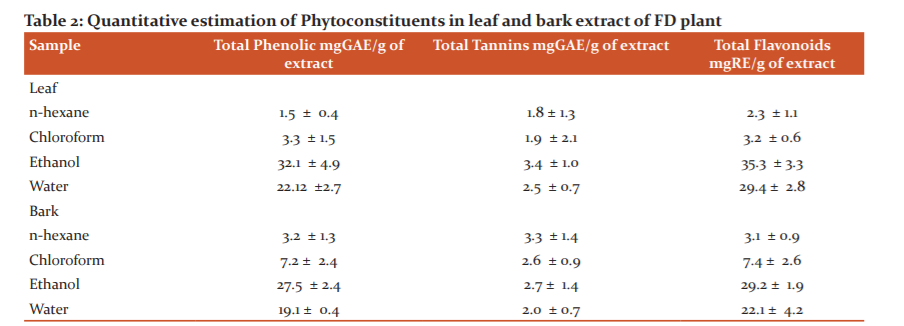
Values are mean of 3 replicate determinations + SD. GAE- Gallic acid equivalent, RE- rutin equivalent.
3.3. Acute Toxicity Study
The acute toxicity studies of SI leaves extract was carried out as per OECD guideline no. 423. The limit test dose used for the study was 2000 mg/kg. There was no gross evidence of any abnormality observed up to a period of 4-6 hrs or mortality up to a period of 24hrs at the maximum tolerated dose level of 2000 mg/kg body weight p.o. Results demonstrated that animals did not display any drug-related changes in behavior, breathing, skin effects, water consumption, and impairment in food intake, temperature, autonomic and neurological. Therefore, the extract seems to be safe at a dose level of 2000 mg/kg, and the LD50 was considered be >2000 mg/kg.
3.4 Effect of ethanolic leaf extract of SI (ELESI) on body weight
No difference in the initial body weight was observed in any experimental group. Two-way ANOVA revealed that STZ subjected rats gained less body weight than normal rats. After a period of 28 days of STZ, a prominent decrease in body weight was found as compared to normal rats (table 3). Higher dose of ELESI and standard drugs significantly prevent the decrease in body weight at 14 and 28 days. Lower dose treatment did not show a pronounced difference in body weight as compared to diabetic control rats.
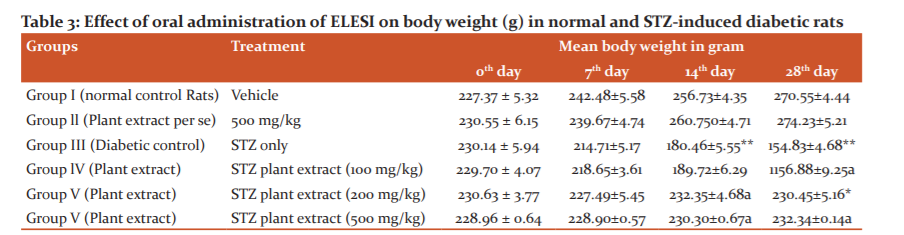
3.5 Effect of extract on Blood Glucose level
The administration of extracts or standard treatment such as glipizide (4 mg/kg, p.o., 2 weeks) to normal rats did not produce any significant per se effects on various parameters assessed at the end of 4 weeks of treatment in the present study. Fasting blood glucose level was significantly elevated (p ? 0.05) after 3 days of STZ treatment with respect to control level. The results showed that rats in control group showed no significant change in blood glucose levels is observed at 7, 14, 21 and 28 day of experiment (table 4). No significant changes in blood glucose levels were observed after oral administration of 500 mg/kgbwt of SI in normal animals at 28 day of experiment when compared to control. However, treatment with single dose of STZ at a dose of 180 mg/kgbwt after 3 days caused significant increase (p ? 0.05) in blood glucose levels of rats. Whereas, oral administration of 100 mg/kgbwt, 200mg/kgbwt and 500mg/kgbwt of ethanolic leaf extract of SI for 28 days showed significant reduction (p?0.05) in blood glucose levels when compared to STZ treated group (Table 6). Treatment with Glipizide (4 mg/kg body weight, 4 weeks) significant decreased the glucose level at 7, 14, 21 and 28 days when compared with diabetic control rats.
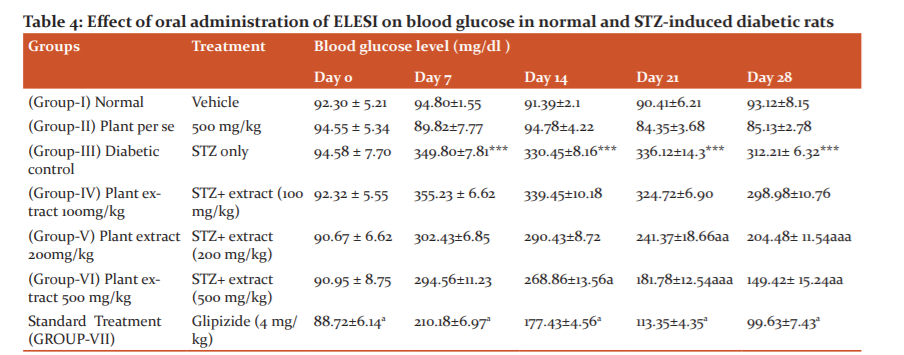
Effect of extract on serum glucose. Data are mean ± SEM; Data were analyzed using one-way ANOVA followed by Tukey’s multiple test; *p<0.01 as compared to Vehicle control Group; ap<0.05 as compared to Diabetic control group.
3.6 Effect of ELEFD on serum lipid profile
The results showed that the serum TC, TG, LDL and VLDL levels increased to 167.39 mg/dl, 178.13 mg/dl, 103.54 mg/dl, 35.62 mg/dl and decreased level of HDL (28.22 mg/dl) in STZ-induced diabetic rats. When treated with standard drug glipizide lipid levels were reduced significantly (table 5). Among the plant extract high doses of SI (200 and 500mg/kg) worked effectively and significantly altered the lipid parameters in diabetic rats in dose-dependent manner. However, lower dose did not produced significant effect on the TC, TG, HDL, LDL and VLDL levels in diabetic rats.
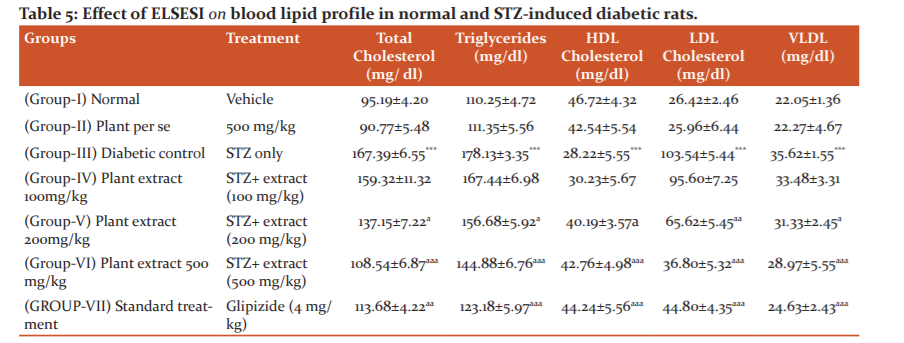
Effect of extract on serum lipid. Data are mean ± SEM; Data were analyzed using one-way ANOVA followed by Tukey’s multiple test; *P<0.01 as compared to Vehicle control Group; aP<0.05 as compared to Diabetic control group.
3.7 Effect of plant extracts on serum oxidative/antioxidant parameters
The levels of oxidative markers detected in the serum of normal and diabetic control rats are summarized in table 6. STZ administration resulted in a profound increase in TBARS levels, as compared with normal controls. Chronic administration of 200mg/kg and 500mg/kg of plant extracts of SI significantly reduced the elevated levels of TBARS in serum of diabetic rats in comparison to the levels observed in vehicle-treated diabetic control rats. However, plant extract at lower dose (100mg/kg) did not notably in? uence the TBARS level in STZ rats.
The anti-oxidant enzyme status in serum samples was assessed by measurements of Glutathione (GSH), Superoxide dismutase (SOD), catalase (CAT) levels. Serum GSH, SOD and CAT levels were decreased in diabetic rats when compared with normal control group, indicative of impairment in anti-oxidant status. Treatment with plant extract of SI (200 & 500 mg/kg,) and glipizide restored serum GSH, SOD and CAT levels as compared to control group (table 6). Further, lower dose (100 mg/kg, p.o.) did not produce any effect on the anti-oxidant enzyme levels in diabetic rats.
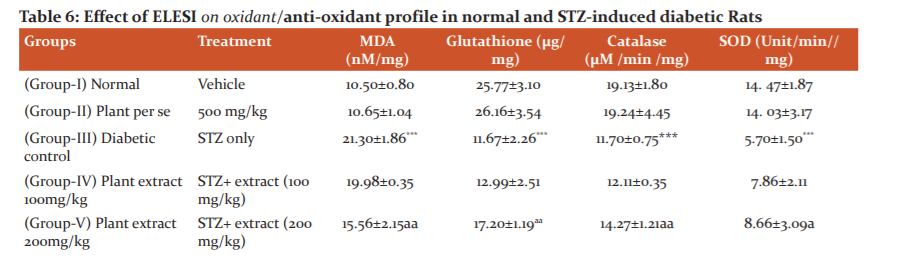
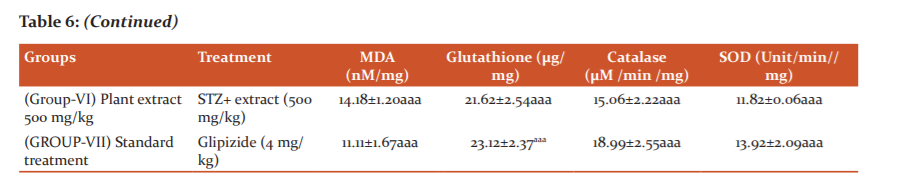
Effect of extract on oxidant/anti-oxidant profile. Data are mean ± SEM; Data were analyzed using one-way ANOVA followed by Tukey’s multiple tests; *P<0.01 as compared to Vehicle control Group; aP<0.05 as compared to Diabetic control group.
4. Discussion
Medicinal plants have a long-standing history in many indigenous communities and continue to provide useful tools for treating various diseases. The practices of traditional medicine are based on hundreds of years of belief and observations, which predate the development and spread of modern medicine.39 It is reported that compounding of highly standardized herbal products with reference to chemical composition and pharmacological activity is considered as an important approach in this field. Considering the above-mentioned aspects, this comprehensive study examines the prominent features such as pharmacognostic, Phytochemical and pharmacological activity of leaves and bark of SI plant.
In present study, the results of the phytochemical screening revealed that, all the bark and leaf extract of the SI plant showed the presence of flavonoids, Saponins, steroids, alkaloids, tannins, phenolic compounds, triterpenoids and carbohydrates whereas, glycosides, anthraquinones, and reducing sugars were absent. Moreover, quantitative estimation of total tannins, total phenolic and total flavonoids compounds were also performed in the leaf and bark of all three plants. The ELESI shows the presence of a higher amount of total phenol, total flavonoid and tannins components as compared to other extracts. This report eventually justifies the preliminary phytochemical studies and also helps in finding suitable extracts for the pharmacological study.
Extracts of SI plant was further investigated for their beneficial effects in the management of STZ-induced diabetes. In the present study diabetes was induced by the administration of STZ. The results, based on biochemical parameters, were compared with normal control, diabetic control and positive control rats treated with glibenclamide. The result of the present study showed significant changes in the biochemical parameter of the experimentally induced diabetes. In support to earlier reports, increased fasting blood glucose (FBG) level was seen in STZ induced diabetic rats compared to the control group.40,41 FBG was significantly attenuated by treatment with all three plant extracts ELESI, which is consistent with our previous report. Plant extracts normalize the glucose level similar to the standard drug (glimepiride) and exhibited as a potent anti-diabetic effect. The decrease in the level of FBG might be attributed to the insulin secretion from residual pancreatic cells or regeneration β-cell. Moreover, an increase in insulin secretion from remnant β-cells and an increase in the peripheral utilization of glucose may also contribute to the anti-hyperglycemic action of plants. So, this study divulged the association between glucose level, and insulin levels.
Along with hyperglycemia, induction of diabetes with STZ is associated with characteristic weight loss. Oral administration of ELESI improved body weight in diabetic rats. The decreased body weight was due to protein metabolism and muscle wasting. After treatment with plant extracts, diabetic animals showed improvement in body weight. This increased body weight might be linked to insulin secretion which improves glucose level in diabetic animals.
The correlation in hyperglycemia and dyslipidemia is well known.42,43 Increase level of lipids leads to atherosclerosis which may cause diabetes and complications of diabetic.43 In our study, a significant increase in TC, TG, VLDL and LDL level was observed along with a significant reduction in HDL level in diabetic rats. Administration of plant extracts effectively increased serum HDL and reduced the level of TC, TG, LDL and VLDL cholesterol. Interestingly, plant extracts at higher doses showed quite similar results as to glimepiride treated group in the reduction of TC, TG, LDL and VLDL indicating equivalent hypolipidemic activity similar to available standard drugs. Moreover, in the case of HDL, the highest dose of both the extracts showed more pronounced effects as compared to standard drug glimepiride.44,45,46
Moreover, the etiology of diabetes involves various factors like increased oxygen free radical, alteration in antioxidant enzymes, nonenzymatic protein glycosylation, impaired glutathione metabolism and lipid peroxidation.47 STZ-induced hyperglycemia elevates ROS generation and depresses antioxidant defense resulting in cellular disruption and increased lipid peroxidation.48 Thus, oxidative stress can be diminished via the diminution of free radical generation.
Also, the potential in-vitro antioxidant activity of plant extracts were estimated by DPPH radical scavenging and reducing power activity method. The WBESI showed dose-dependent increases in scavenging activity on free radicals in our present study.
Polyphenolic compounds such as flavonoids, phenolic acids and tannins are considered to be the major contributors to the antioxidant activity of fruits and vegetables of medicinal plants. Phenol and phenolic compounds such as flavonoids have been shown to possess significant antioxidant activities and their effects on human nutrition and health are considerable.49 Hence, the leaf and bark extracts of FD, SI and CM could be a good source of antioxidants.
In our study various antioxidant enzyme level like SOD, CAT, and GSH were significantly decreased in diabetic rats, whereas the level of TBARS increased significantly It is previously reported that the activity of antioxidant enzymes is reduced in serum and tissue homogenate of diabetic rats.50 In this study, treatment with ethanolic leaf extract of all three plants reduced TBARS level and increased SOD, CAT as well as GSH levels, due to its potential antioxidant activity. Reduction in the level of TBARS after treatment shows the effective antioxidant activity of ELESI. Thus, cumulative results show the antidiabetic potential of plans.
Conclusion
The study carried out the evaluation of the anti-diabetic activity of Sophora interrupta by using streptozotocin-induced diabetic rat model and revels that the ethanolic extract of SI showed significant antioxidant, antihyperlipidemic and antidiabetic activity suggesting an alternate and promising therapeutic regimen in the treatment of diabetes and secondary complications.
Acknowledgment: Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors/editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
Conflict of Interest: The authors declare(s) that there is no conflict of interest
Source of funding: Nil
Authors contribution:
Selection of plant material- Faheem I.P and Sarah Priya
Preliminary phytochemical screening-Mohsina F.P and Sarah Priya
Quantitative estimation of Total phenolic, Flavonoids and Tannins-Faheem I.P and Dr. B.Gopalakrishna
Acute toxicity studies-Faheem I.P and Dr. B.Gopalakrishna
Designing of experimental protocol- Faheem I.P, Mohsina F.P and Dr. B.Gopalakrishna
Biochemical estimation- Faheem I.P, Mohsina F.P
References:
-
Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med.1998 Jul;15(7):539-53.
-
Patel P, Macerollo A. Diabetes mellitus: diagnosis and screening. Am Fam Physician. 2010 Apr 1;81(7):863-70.
-
Yoganarasimhan SN. Medicinal plants of India: Tamil Nadu. CyberMedia; 2000.
-
Rituparna M, Neeraj K A. Practitioners section-Atherosclerosis in diabetes mellitus: Role of inflammation. Indian J Med Sci.2007 May 1;61(5):292-306.
-
Pan D, Zhang D, Wu J, Chen C, Xu Z, Yang H, Zhou P. A novel proteoglycan from Ganodermalucidum fruiting bodies protects kidney function and ameliorates diabetic nephropathy via its antioxidant activity in C57BL/6 db/db mice. Food ChemToxicol. 2014 Jan 1;63:111-8.
-
Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004 May 1;27(5):1047-53.
-
Watanabe RM. The genetics of insulin resistance: where’s Waldo?. Current diabetes reports. 2010 Dec 1;10(6):476-84.
-
Pandey A, Tripathi P, Pandey R, Srivastava R, Goswami S. Alternative therapies useful in the management of diabetes: A systematic review. J Pharm Bioallied Sci. 2011 Oct;3(4):504.
-
Greenspan FS, Gardner DG. Basic and clinical endocrinology 2001. p. 330–5.
-
Surendran S, Eswaran B, Vijayakumar M, Ch&, Rao V. In vitro and in vivo hepatoprotective activity of Cissampelospareira against carbon-tetrachloride induced hepatic damage. Indian J Exp Biol. 2011;49: 939–45.
-
Shahzadi I, Hassan A, Khan UW, Shah MM. Evaluating biological activities of the seed extracts from Tagetesminuta L. found in Northern Pakistan. J. med plant res. 2010 Oct 18;4(20):2108-12.
-
Ekor M, Osonuga OA, Odewabi AO, Bakre AG, Oritogun KS. Toxicity evaluation of Yoyo cleanser bitters and fields Swedish bitters herbal preparations following sub-chronic administration in rats. Am. J. Pharmacol. Toxicol. 2010;5:159-66.
-
Karimi A, Majlesi M, Rafieian-Kopaei M. Herbal versus synthetic drugs; beliefs and facts. J neuropharmacology. 2015;4(1):27–30.
-
Butler MS. The role of natural product chemistry in drug discovery. J Nat Prod. 2004 Dec 28;67(12):2141-53.
-
Farnsworth NR. Screening plants for new medicines. Biodiversity. 1988 Jan 15;15(3):81-99.
-
Samanta SK. Modulation of male infertility by ayurvedic drugs. Int Sem-Trad med 1992 Nov 7.
-
Hemamalini K, Bhargav A. Evaluation of Phytochemical and pharmacological activity of methanolic extract of Sophorainterrupta. Indo Am J Pharm Res. 2013;3:6381-90.
-
Manjula CH, Ammani K. Phytochemical analysis and pharmacological importance of Sophorainterrupta leave. International J. Res. Pharm and Biomed. Sci. 2012;3(4):1798-804.
-
Wink M. Evolutionary advantage and molecular modes of action of multi-component mixtures used in phytomedicine. Curr Drug Metab. 2008 Dec 1;9(10):996-1009.
-
Hemamalini K, Suvidha S, Bhargav A, Vasireddy U. Evaluation of anti-ulcer activity of methanolic extracts of Kigeliaafricana, Sopharainterrupta and Holopteleaintegrifolia leaves in experimental rats. International J. Curr. Pharm. Res. 2012;4(4):61-6.
-
Munikishore R, Rammohan A, Padmaja A, Gunasekar D, Deville A, Bodo B. A new O-prenylated flavonol from the roots of Sophorainterrupta. Nat Prod Res. 2013 Oct 1;27(20):1823-6.
-
Harborne JB. Methods of plant analysis. InPhytochemical methods 1984 (pp. 1-36). Springer, Dordrecht.
-
Evans WC. Trease and evans' pharmacognosy E-book. Elsevier Health Sciences; 2009 May 27.
-
Hahn-Deinstrop E. Applied thin-layer chromatography: best practice and avoidance of mistakes. John Wiley & Sons; 2007 Feb 27.
-
Brain KR, Turner TD. The practical evaluation of phytopharmaceuticals. Bristol: Wright-Scientechnica; 1975.
-
Daniel M, Daniel M. Methods in plant chemistry and Economic Botany. Kalyani Pubs.; 1991.
-
Shriner RL, Hermann CK, Morrill TC, Curtin DY, Fuson RC. The systematic identification of organic compounds. John Wiley & Sons; 2003 Aug 19.
-
Al-Yahya MA, Al-Farhan AH, Adam SE. Preliminary toxicity study on the individual and combined effects of Citrulluscolocynthis and Nerium oleander in rats. Fitoterapia. 2000 Aug 1;71(4):385-91.
-
Gallo MB, Sarachine MJ. Biological activities of lupeol. Int. J. Biomed. Pharm. Sci. 2009 Oct;3(1):46-66.
-
Allain CC, Poon LS, Chan CS, Richmond WF, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974 Apr 1;20(4):470-5.
-
Werner M, Gabrielson DG, Eastman J. Ultramicro determination of serum triglycerides by bioluminescent assay. Clin Chem. 1981 Feb 1;27(2):268-71.
-
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without the use of the preparative ultracentrifuge. Clin Chem.. 1972 Jun 1;18(6):499-502.
-
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by the thiobarbituric acid reaction.Anal Biochem. 1979 Jun 1;95(2):351-8.
-
Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972 May 25;247(10):3170-5.
-
Ellman GL. Tissue sulfhydryl groups. Arch BiochemBiophys. 1959 May 1;82(1):70-7.
-
Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972 Jun 1;47(2):389-94.
-
Trease and Evaans. Trease and Evans Pharmacognosy, International Edition E-Book - William Charles Evans
-
Harborne AJ. Phytochemical methods a guide to modern techniques of plant analysis. springer science & business media; 1998 Apr 30.
-
Jeyaprakash K, Ayyanar M, Geetha KN, Sekar T. Traditional uses of medicinal plants among the tribal people in Theni District (Western Ghats), Southern India. Asian Pac. J. Trop Biomed. 2011 Sep 1;1(1):S20-5.
-
Kaur N, Kishore L, Singh R. Antidiabetic effect of new chromane isolated from Dilleniaindica L. leaves in streptozotocin-induced diabetic rats. J. Func. Foods. 2016 Apr 1;22:547-55.
-
Madhavan V, Joshi R, Murali A, Yoganarasimhan SN. Antidiabetic activity of Curculigoorchioides. Root tuber. Pharmaceutical Biology. 2007 Jan 1;45(1):18-21.
-
Lönn ME, Dennis JM, Stocker R. Actions of “antioxidants” in the protection against atherosclerosis. Free RadicBiol Med. 2012 Aug 15;53(4):863-84.
-
Thomas M, Reddy NG. EVALUATION OF ANTI-HYPERLIPDEMIC EFFECT OF BACOPA MONNIERI LINN. IN ATHEROGENIC DIET-INDUCED HYPERLIPIDEMIC RATS. EMCare Cover J PHARMANEST AnInt J Adv Pharm Sci. 5:5.
-
Song Y, Manson JE, Tinker L, Rifai N, Cook NR, Hu FB, Hotamisligil GS, Ridker PM, Rodriguez BL, Margolis KL, Oberman A. Circulating levels of endothelial adhesion molecules and risk of diabetes in an ethnically diverse cohort of women. Diabetes. 2007 Jul 1;56(7):1898-904.
-
Nogueira JP, Brites FD. Rol del enterocito en la dislipemia de la resistenciainsulínica. Endocrinol Nutr.2013 Apr 1;60(4):179-89.
-
Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat ClinPractEndocrinolMetab. 2009 Mar;5(3):150-9.
-
Yazdanparast R, Ardestani A, Jamshidi S. Experimental diabetes treated with Achilleasantolina: effect on pancreatic oxidative parameters. J Ethnopharmacol. 2007 May 30;112(1):13-8.
-
Cameron NE, Cotter MA. Potential therapeutic approaches to the treatment or prevention of diabetic neuropathy: evidence from experimental studies. Diabet Med. 1993 Aug 9;10(7):593-605.
-
Kessler M, Ubeaud G, Jung L. Anti?and pro?oxidant activity of rutin and quercetin derivatives. J Pharm Pharmacol. 2003 Jan;55(1):131-42.
-
Kishore L, Kaur N, Singh R. Effect of Kaempferol isolated from seeds of Eruca sativa on changes of pain sensitivity in Streptozotocin-induced diabetic neuropathy. Inflammopharmacology. 2018 Aug;26(4):993-1003.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License