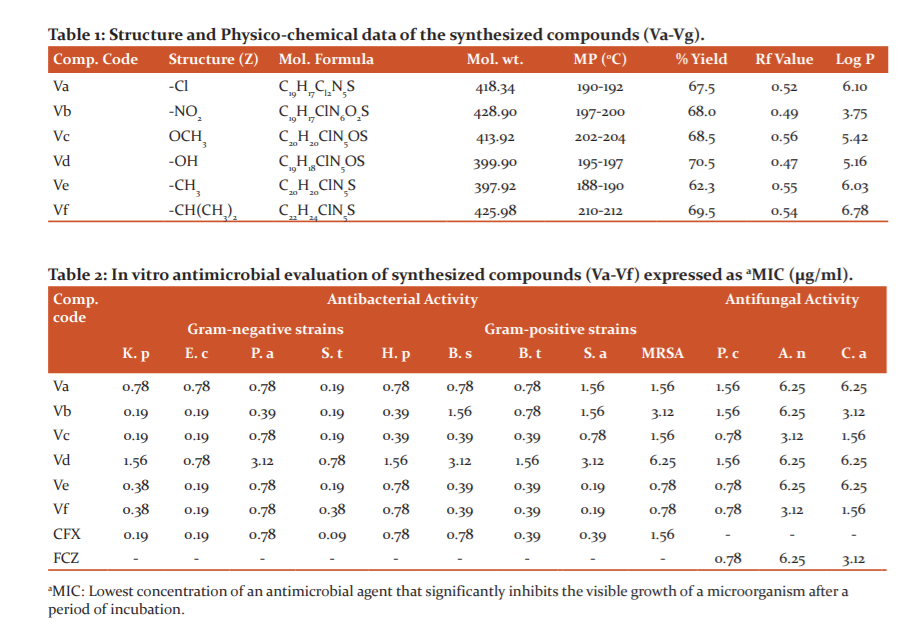
IJCRR - 13(13), July, 2021
Pages: 45-55
Date of Publication: 05-Jul-2021
Print Article
Download XML Download PDF
Synthesis, Characterization, In-Vitro Antimicrobial Evaluation and Molecular Docking Studies of Aromatic Aldehydes Substituted Thiosemicarbazide Quinoxaline Derivatives
Author: Bipin Bihari, Girendra Kumar Gautam, Akash Ved
Category: Healthcare
Abstract:Background: In the present research work a series of novel quinoxaline thiosemicarbazide derivatives were synthesized by substitution of some aromatic aldehydes and their antimicrobial evaluation against various microbial strains with molecular docking studies. Methods: Lead molecule (1E, 4E)-1-(7-chloro-3-isopropyl quinoxaline-2(1H)-ylidene) thiosemicarbazide was synthesized and condensed with various aromatic aldehydes to synthesize derivatives. All derivatives (Va-Vf) were characterized by IR., NMR & Mass spectroscopy. The synthesized derivatives were evaluated in vitro for antibacterial and antifungal activities using the agar dilution method. Molecular docking studies of the derivatives were performed against E.coli DNA gyrase B and topoisomerase IV to find out essential binding sites against target protein PDB: 1AJ6 and 1S14 respectively. Results: Compounds Vb, Vc, Ve&Vf exhibited potent antibacterial and antifungal activity. Compounds, Vb and Vc were found to exhibit more potent activity against Gram \?Ve, bacterial strains at MIC 0.19 µg/ml whereas compound Ve and Vf showed potent activity against Gram +Ve bacterial strains and fungal strains at MIC 0.19 µg/ml and 0.78 µg/ml respectively. The docking studies revealed that all the compounds exhibit extensive binding to the active pockets of E.coli DNA gyrase B and topoisomerase IV. The compound Vb and Ve exhibit interactive binding energy -8.0 and -8.3 Kcal/mole to the active pocket site of E.coli DNA gyrase and -8.2 and 7.9 Kcal/mole to the active pocket site of topoisomerase IV respectively. Conclusion: In terms of SAR study, it was revealed that the activity profile against microbial strains was altered with electronic effects like electron-withdrawing or donating substitutions in aromatic aldehydes substituted quinoxaline thiosemicarbazide derivatives.
Keywords: Antimicrobial, Aromatic aldehydes, E.coli DNA gyrase B, E.coli Topoisomerase IV, Quinoxaline, Thiosemicarbazide
Full Text:
INTRODUCTION:
Struggling for the development of an antimicrobial drug is a vital global issue due to the rapid development of resistance to currently used antimicrobial drugs, the emergence of new microbial infections and the existence of chronic microbial infections.1Bacteria may obtain resistance through a variety of mechanisms such as by spontaneous mutations or acquisition of genetic material from other resistant organisms or modifying binding sites or production of enzymes that inactivate antimicrobial agents or altering in outer membrane protein channel that the drugs require for cell entry.2
Quinoxaline and its derivatives are important nitrogen-containing benzo-hetero cyclic compounds.3 Substituted quinoxaline derivatives are extensively employed in the building blocks of various pharmacologically active compounds. They exhibit a broad range of pharmacological activities such as antibacterial4,5,6 antifungal7,8, antitubercular9, antimalarial 10,11, antileishmanial12, anticancer13,14 and antidepressant15,16. Also, quinoxaline derivatives reported for antioxidant17, antimycobacterial18, antithrombotic19 and topoisomerase inhibition activity.20
Over the past few decades, an immense interest of researchers has been focused on thiosemicarbazide pharmacophore due to their wide range of synthetic and analytical applications and pharmacological activities21,22. Thiosemicarbazide derivatives were synthesized and studied for various pharmacological activities such as antiviral23,24, anticancer 25, antitumor26,27, antiamoebic28,29, antiproliferative30, antidiabetic[31], anti-HIV32, anti-tubercular activities33. Recently, many thiosemicarbazide derivatives were synthesized and studied for their antibacterial and antifungal activity.34,35,36,37
DNA gyrase and topoisomerase IV are essential enzymes that display crucial roles in biological processes of bacterial growth such as replication, transcription, recombination repair and chromatin remodeling.38,39Nitrogen containing heterocyclic may be potential antibacterial drugs that inhibit bacterial topoisomerases40such as triazoles41, quinolones42, oxazolopyridines, amino pyrazinamides and pyrazole.43In continuation of our search, it was found that certain thiosemicarbazide derivatives evaluated as inhibitors of DNA gyrase and topoisomerase II of S. aureus and E. coli.44,45
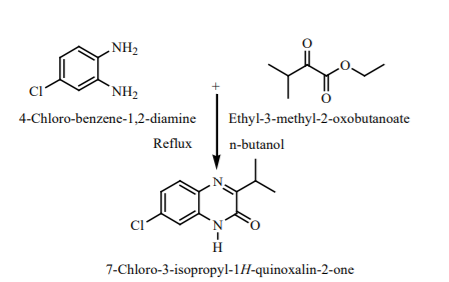
Based on the aforementioned facts, we decided to synthesize some novel quinoxaline hybrid thiosemicarbazide derivatives incorporated with different aromatic aldehydes through imine linkage in the thiosemicarbazide nucleus. All the synthesized compounds were screened in vitro for antibacterial and antifungal activities against various strains. In addition, to understand the mechanism of action and binding activity, molecular docking studies were performed against two kinases, E. coli DNA gyrase B and E. coli topoisomerase IV against target protein PDB: 1AJ6 and 1S14 respectively46,47. Computational studies were performed to analyze binding and orientation patterns of the ligands with amino acids against target protein (PDB: 1AJ6 and 1S14)
EXPERIMENTAL
Materials and methods
All the reagents and the solvents used in the research work were of synthetic reagent grade and obtained from Qualigens Ltd. (Fisher Scientific), Ranbaxy, and Fine Chemicals Ltd. India. Muller-Hinton and Sabouraud dextrose agar were obtained from Hi-Media Ltd. India. The bacterial and fungal strains were provided by the Department of Biotechnology of Saroj Institute of Technology & Management, Lucknow, India.
The Progress of reactions and purity of derivatives were monitored by ascending thin layer chromatography on precoated silica gel-G sheets (E. Merck and Co.).Column chromatography was performed over silica gel (60-120 Mesh) obtained from QualigensTM(India). The percentage of yield, Rf values, melting points, and spectral analysis are given for various purified compounds. Yields are presented for crude products. Log P values for synthesized compounds were calculated by using Chem Draw Ultra 10.0.
Melting points were determined by using the Digital Elico melting point apparatus. Infra-red spectra were measured on a Perkin-Elmer FT-IR RXI Spectrophotometer. 1HNMR spectra were reported on a Bruker DPX-300 Spectrometer (300 MHz) using DMSO-D6 as a solvent and tetramethylsilane (TMS) as an internal reference standard. Electron Spinning Ionization Mass spectra (ESI-MS) were obtained on the JEOL SX 102 spectrometer. Elemental analysis was determined on an Elemental Vario EL-III elemental analyzer. The structure of molecules was confirmed based on spectral data.
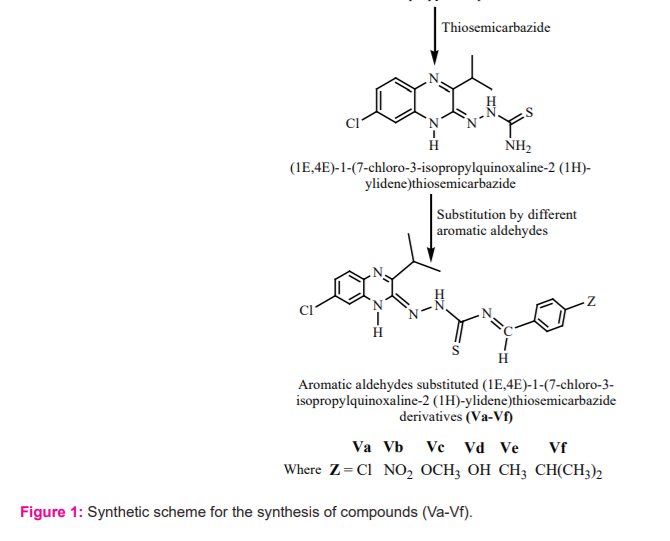
A series of aromatic aldehydes substituted (1E, 4E)-1-(7-chloro-3-isopropyl quinoxaline-2(1H)-ylidene) thiosemicarbazide derivatives were synthesized according to the respective scheme (Figure 1).
Synthesis of 7-Chloro-3-isopropyl-1H-quinoxaline-2-one(III):
4-Chlorobenzene-1, 2-diamine (I) (21.3 g, 0.15 M) was dissolved in n-butanol (300 ml) and warmed. Ethyl dimethyl pyruvate (II) (21.6 g, 0.15 M) was solubilized separately in n-butanol (150 ml) and added to the former solution with constant stirring. The reaction mixture was refluxed for about 1 hour 30 minutes on the water bath. The reaction mixture was allowed to cool, obtained crystals, which were allowed for filtration, washed and purified by recrystallization from ethanol to obtain the white crystals of 7-chloro-3-isopropyl-1H-quinoxaline-2-one (III). The completion of the reaction and purity of the compound was checked by a single spot TLC.
Yield: 89.5%; m.p.225-228oC; Mol. Formula:C11H11ClN2O; Mol. Wt:222.67; IR (KBr, cm-1): 3465 (NH str.), 3102(C-H sp2 str.), 1659(C=N str.), 1605(C=C aromatic str.), 1372(CH (CH3)2str.). 1042(C-Cl str.), 1690(C=O str.); 1H- NMR(300 MHz, DMSO-d6) δ(ppm): 10.15(S, 1H, NH), 8.06(S, 1H, Ar. H), 7.25 -7.28(d, 1H, Ar. H), 6.97-7.05(d, 1H, Ar. H), 2.25-2.43(m, 1H, CH, i-pr.), 1.63(s, 6H, -(CH3)2); ESI-MASS: m/z[M+1]+223.19;Anal. Calculated for (C11H11ClN2O): C, 59.33; H, 4.98; N, 12.58;Found: C, 59.29; H, 5.02; N, 12.52.
Synthesis of (1E,4E)-1-(7-chloro-3-isopropylquinoxalin-2(1H)-ylidene) thiosemicarbazide(IV):
7-Chloro-3-isopropyl-1H-quinoxaline-2-one (III) (22 g, 0.10 M) was dissolved in ethanol (350 ml) and added thiosemicarbazide (9 g, 0.10 M). The reaction mixture was stirred and refluxed for 4 hours. The reaction mixture was allowed to cool at room temperature, obtained crystals. The crystals were collected by filtration, washed and purified by recrystallization from ethanol to yield white crystals of (1E, 4E)-1-(7-chloro-3-isopropyl quinoxaline-2(1H)-ylidene) thiosemicarbazide (IV). The completion of the reaction and purity of the compound was checked by a single spot TLC.
Yield: 85.5 %; m.p. 218--222 oC; Mol. Formula: C12H14ClN5S; Mol. Wt: 295.79; IR (KBr, cm-1): 3442(NH str.), 3066(C-H sp2 str.), 3002(N-H2str.), 1654(C=N str.), 1602(C=C aromatic str.), 1361(CH(CH3)2str.). 1037(C-Clstr.);1H-NMR(300MHz,DMSO-d6)δ(ppm):10.02(S,1H,NH),9.62(S,1H,NH),8.09(S,1H,Ar.H),7.26-7.34(d,1H,Ar.H),6.96-6.98(d,1H,Ar.H),4.99(S,2H,NH2),2.18-2.64(m,1H,CH,i-pr.),1.59(d,6H,-(CH3)2);ESI-MASS:m/z[M+1]+296.09;Anal.CalculatedforC12H14ClN5S:C,48.73;H,4.77;N,23.68;S,10.84;Found:C,48.69;H,4.72;N,23.74.
General procedure for the synthesis of a series of different aromatic aldehydes substituted quinoxalinethiosemicarbazide derivatives (Va -Vf):
A typical procedure is described here for the synthesis of a series of different aromatic aldehyde substituted quinoxaline thiosemicarbazide derivatives. In this step N'-(7-chloro-3-isopropyl-1H-quinoxaline-2-ylidine) thiosemicarbazide (IV) (0.01 mmol) was refluxed with different aromatic aldehydes (0.01 mol), in methanol (50 ml) and added glacial acetic acid (6-8 drops) for 4-5 hours. The progress of the reaction was monitored by TLC on silica-gel 60 plates until a distinct spot of the product was obtained. At the end of the reaction, the crude precipitate was filtered and recrystallized with methanol. The final product thus obtained was chromatographed on silica gel (60-120 mesh), using solvent system chloroform: methanol (3:1) as eluent to furnish pure compounds Va-Vf.
(1E,4E)-1-(7-chloro-3-isopropylquinoxalin-2(1H)-ylidene)-4-(1-(4-chlorophenyl) methylidene) thiosemicarbazide(Va):
Yield: 67.5 %; m.p.190-192 oC; Mol. Formula:C19H17Cl2N5S;Mol. Wt:418.34; IR (KBr, cm-1): 3215(N-H str.), 2933(C-H sp2 str. aromatic), 2875(C-H sp2 str. alkyl), 1599(C=N str.), 1572(C=C aromatic str.), 1337(CH (CH3)2 str.), 1235(C=S str.), 785(C-Cl str.), 1110,1057,1005( aromatic C-H in plane bending), 668,614,519( aromatic C-H out plane bending); 1H NMR (DMSO-d6): δ 10.89(s,1H, N-NH), 10.68(s,1H, NH), 9.15(s, 1H, N=CH), 7.28(s,2H, Ar. H), 7.24-7.27(d, 1H, Ar. H-8), 7.13-7.16(d, 1H, Ar. H-5), 7.01-7.04(d, 1H, ArH-6), 6.95-6.98(s,2H, Ar. H), 2.21-2.24(m, 1H, CH i-pr.), 1.95(s,6H,-(CH3)2); ESI-MASS: m/z [M+1]+ 419.07;Anal. Calculated. for C19H17Cl2N5S:C, 54.55; H, 4.10; N, 16.74; S, 7.66; Found: C, 54.52; H, 4.14; N, 16.73.
(1E,4E)-1-(7-chloro-3-isopropylquinoxalin-2(1H)-ylidene)-4-(1-(4-nitrophenyl) methylidene) thiosemicarbazide(Vb):
Yield: 68 %; m.p.197-200 oC; Mol. Formula:C19H17ClN6O2S;Mol. Wt:428.90; IR (KBr, cm-1): 3402(N-H str.), 2939(C-H sp2 str. aromatic), 2864(C-H sp2 str. alkyl), 1607(C=N str.), 1579(C=C aromatic str.), 1398(CH (CH3)2 str.), 1231 (C=S str.), 747 (C-Cl str.), 1147,1105,1054 ( aromatic C-H in plane bending), 850,668,610,560 ( aromatic C-H out plane bending); 1H NMR (DMSO-d6): δ 11.02 (s,1H, N-NH), 10.60 (s,1H, NH), 9.31 (s, 1H, N=CH), 7.86-7.89 (d,2H, Ar. H), 7.82-7.85 (d, 1H, Ar. H-8), 7.59-7.62 (d, 1H, Ar. H-5), 7.28-7.34 (d, 1H, Ar. H-6), 6.96-6.98 (d,2H, Ar. H), 2.17-2.23 (m, 1H, CH,i-pr.), 1.30 (s,6H,-(CH3)2); ESI-MASS: m/z [M+2]+ 430.15;Anal. Calculated. for C19H17ClN6O2S: C, 53.21; H, 4.00; N, 19.59; S, 7.46; Found: C, 53.24; H, 4.5; N, 19.56.
(1E,4E)-1-(7-chloro-3-isopropylquinoxalin-2(1H)-ylidene)-4-(1-(4-methoxyphenyl) methylidene) thiosemicarbazide(Vc):
Yield: 68.5 %; m.p.202-204oC; Mol. Formula:C20H20ClN5OS;Mol. Wt:413.92; IR (KBr, cm-1): 3380(N-H str.), 3236 (C-H sp2 str. aromatic), 3097 (C-H sp2 str. alkyl), 1611 (C=N str.), 1576 (C=C aromatic str.), 1394 (CH (CH3)2 str.), 1224(C=S str.), 779 (C-Cl str.), 1140,1052,979 ( aromatic C-H in plane bending), 904,668,556 ( aromatic C-H out plane bending); 1H NMR (DMSO-d6): δ 10.60 (s,1H, N-NH), 10.51(s,1H, NH), 9.05 (s, 1H, N=CH), 7.71-7.74 (d,2H, Ar. H), 7.50-7.53 (d, 1H, Ar. H-8), 7.28-7.35 (d, 1H, Ar. H-5), 6.94-6.97 (d, 1H, ArH-6), 6.72-6.76 (d,2H, Ar. H), 2.46-2.53 (m, 1H, CH,i-pr.), 1.56 (s, 3H, -OCH3), 1.22 (s,6H,-(CH3)2); ESI-MASS: m/z [M+1]+ 415.02;Anal. Calculated. for C20H20ClN5OS: C, 58.03; H, 4.87; N, 16.92; S, 7.75;Found: C, 58.07; H, 4.92; N, 16.88.
(1E,4E)-1-(7-chloro-3-isopropylquinoxalin-2(1H)-ylidene)-4-(1-(4-hydroxyphenyl) methylidene) thiosemicarbazide(Vd):
Yield: 70.5 %; m.p.195-197oC; Mol. Formula:C19H18ClN5OS;Mol. Wt:399.90; IR (KBr, cm-1):3430 (O-H str.), 3395 (N-H str.), 2924 (C-H sp2 str. aromatic), 2839 (C-H sp2 str. alkyl), 1601 (C=N str.), 1569 (C=C aromatic str.), 1372 (CH (CH3)2 str.), 1222 (C=S str.), 738 (C-Cl str.), 1145,1115,1044 ( aromatic C-H in plane bending), 843,657,602,548 ( aromatic C-H out plane bending); 1H NMR (DMSO-d6): δ 10.97 (s,1H, N-NH), 10.56 (s,1H, NH), 10.05 (s, 1H, OH), 9.67 (s, 1H, N=CH), 7.83-7.87 (d,2H, Ar. H), 7.71-7.74 (d, 1H, Ar. H-8), 7.49-7.52 (d, 1H, Ar. H-5), 7.25-7.31 (d, 1H, Ar. H-6), 6.91-6.96 (d,2H, Ar. H), 2.19-2.26 (m, 1H, CH,i-pr.), 1.28 (s,6H,-(CH3)2); ESI-MASS: m/z [M+2]+ 402.08;Anal. Calculated. for C19H18ClN5OS: C, 57.07; H, 4.54; N, 17.51; S,7.46; Found: C, 57.04; H, 4.57; N, 17.48.
(1E,4E)-1-(7-chloro-3-isopropylquinoxalin-2(1H)-ylidene)-4-(1-(4-methylphenyl) methylidene) thiosemicarbazide(Ve):
Yield: 62.3 %; m.p.188-190oC;Mol. Formula: C20H20ClN5S;Mol. Wt: 397.92;IR (KBr, cm-1): 3354 (N-H str.), 2945 (C-H sp2 str. aromatic), 2840 (C-H sp2 str. alkyl), 1625 (C=N str.), 1529 (C=C aromatic str.), 1343 (CH (CH3)2 str.), 1246 (C=S str.), 756 (C-Cl str.). 1173, 1044, 973 (aromatic C-H in plane bending), 835, 644, 593 (aromatic C-H out plane bending).1H NMR(DMSO-d6): δ 11.13 (s,1H, N-NH), 10.70 (s,1H, NH), 9.22 (s, 1H, N=CH), 7.40-7.43 (d,2H, Ar. H), 7.36-7.39 (d, 1H, ArH-8), 6.79-6.81 (d, 1H, ArH-5), 6.58-6.62 (d, 1H, ArH-6), 6.17-6.21 (d,2H, Ar. H), 2.61-2.69 (m, 1H, CH, i-pr.), 1.65 (s, 3H, -CH3), 1.27 (s,6H,-(CH3)2);ESI-MASS: m/z [M+1]+ 399.14;Anal. Calculated. for C20H20ClN5S: C, 60.37; H, 5.07; N, 17.60; S, 8.06;Found:C, 60.41; H, 5.11; N, 17.57.
(1E,4E)-1-(7-chloro-3-isopropylquinoxalin-2(1H)-ylidene)-4-(1-(4-isopropylphenyl) methylidene) thiosemicarbazide(Vf):
Yield: 69.5 %; m.p.210-212oC; Mol. Formula:C22H24ClN5S;Mol. Wt:425.98;IR (KBr, cm-1): 3316(NH str.), 2982 (C-H sp2 aromatic str.), 2808 (C-H sp2 aliphatic str.), 1668 (C=N aromatic str.), 1512 (C=C str.), 1372 (CH (CH3)2 str.), 1342 (C-N str.), 1272 (C=S str.), 789 (C-Cl str.), 1164,1069,978 (aromatic C-H in plane bending), 841,6,98,594 (aromatic C-H in out of plane bending).1H NMR (DMSO-d6):δ 11.15 (s,1H, N-NH), 10.35 (s,1H, NH), 8.61 (s, 1H, N=CH), 7.85-7.88 (d,2H, Ar. H), 7.43-7.47 (d, 1H, Ar. H-8), 7.25-7.25 (d, 1H, Ar. H-5), 6.97-7.01 (d, 1H, ArH-6), 6.71-6.75 (d,2H, Ar. H), 2.25-2.40(m, 2H, CH,i-pr.), 1.34 (s, 6H, (CH3)2 ), 1.30 (s,6H,-(CH3)2); ESI-MASS : m/z [M+1]+ 427.12 Anal. Calculated for C22H30ClN5S : C, 62.03; H, 5.68; N, 16.44; S, 7.53; Found: C, 62.06; H, 5.72; N, 16.41.
ANTI-MICROBIAL EVALUATION
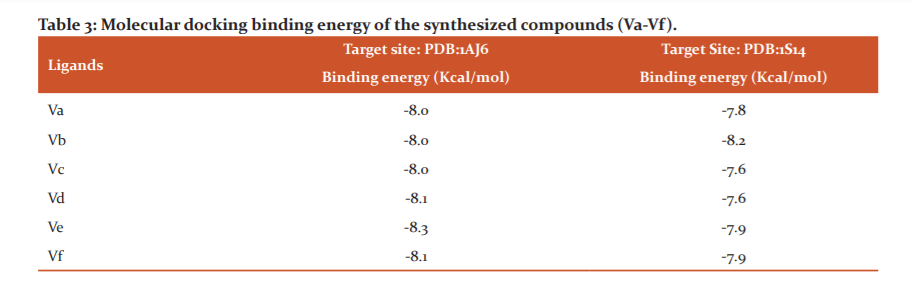
MicrobialStrains: All the synthesized compounds (Va-Vf), were evaluated in vitro for their antibacterial and antifungal activity against Gram-negative bacterial strains such as Klebsielapneumonie(ATCC 15380), Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27893), Salmonella typhi (MTCC 3216), Helicobacter pylori (ATCC 26695) and Gram-positive bacterial strains such as Bacillus subtilis (ATCC 6633), Bacillus thuringiensis (MTCC 714), Staphylococcus aureus (ATCC 25323), methicillin-resistant Staphylococcus aureus (ATCC 3591). Fungal strains used were Penicillium chrysogenum(ATCC 11709), Aspergillus niger(ATCC 9029), Candida albicans (ATCC 90028).
Antimicrobial Assay Methodology: Antimicrobialevaluation of all the synthesized derivatives (Va-Vf), were assayed by using the agar dilution method to determine the minimum inhibitory concentrations (MICs).48. Ciprofloxacin (CFX) and Fluconazole (FCZ) were used as antibacterial and antifungal reference standards, respectively. The range of concentrations of synthesized agents being tested based on the two-fold dilution series (1 mg/L). The dilutions of the synthesized agents and reference drugs were prepared in Mueller-Hinton (MH) agar for bacteria and in Sabouraud dextrose agar for fungi. Each test derivatives (10 mg) were dissolved in 1mL of dimethyl sulfoxide (DMSO) and the solution was diluted with water (9ml). Two-fold dilutions were made with melted Mueller-Hinton and Sabouraud dextrose agar to obtained the necessary concentrations of 100, 50, 25, 12.5, 6.25, 3.13, 1.56, 0.78, 0.39, 0.19, 0.098, 0.049,.0.025, 0.013 and 0.006 µg/ml. The microbial inoculums were prepared by emulsifying overnight colonies from Mueller-Hinton and Sabouraud dextrose agar media in 0.85% saline. The prepared inoculums suspension photometrically adjusted at 600 nm for a cell density comparable to approximately 0.5 McFarland standards (1.5×108 CFU/mL). The suspensions of microorganisms were diluted in 0.85% saline to give 107 CFU/mL for bacteria and 105 CFU/mL for fungi. The plate spot was inoculated with microbial suspensions about 1µl each and incubated at 35-370C for 18-19 hours for bacteria and 28-300C for 50-72 hours for fungi. The minimum inhibitory concentration was observed and determined.
Antibacterial and AntifungalStudy: The synthesized aromatic aldehydes substituted quinoxaline thiosemicarbazide derivatives (Va-Vf) were evaluated for their antibacterial and antifungal activity. Most of the compounds showed excellent to significant activity towards Gram-negative, Gram-positive bacterial and fungal strains. The minimum inhibitory concentration of more active compounds along with Ciprofloxacin ranges from 0.19 – 0.78 µg/ mL for bacteria and with Fluconazole ranges 0.78 – 3.12 µg/ mL for fungal strains. The results of the antibacterial and antifungal activity evaluation are summarized in Table 2.
MOLECULAR DOCKING
Molecular DockingStudies: In today’s globalized world, the molecular docking technique is one of the largely acclaimed structure-based drug design approaches, widely used ever since the early 1980s.49To understand the binding interactions of all the synthesized derivatives were docked into the active site of E.coli DNA gyrase B kinase and E. coli Topoisomerase IV. Crystal structure model of the target (PDB: 1AJ6 and 1S14) were downloaded from worldwide protein data bank (http://www.rcsb.org) and molecular docking studies were performed using the Auto Dock Tool 1.5.6 (ADT) 2011 software (Molecular Graphic Laboratory, The Script Research Institute, U. S. A.), To analyze the docking result and execute the protocol, The Discovery Studio® v17.2.0.16349 software (Client, U. S. A), was employed.
Protein and ligand preparation: The Crystal structure model of the target E.coli DNA gyrase B (PDB code: 1AJ6) and E. Coli Topoisomerase IV ( PDB code: 1S14), with their native ligand novobiocin were downloaded from Protein Data Bank and prepared by the multistep process through the protein preparation menu of the AutoDock (version 1.5.6). The ChemDraw® Ultra 8.0 (Cambridge soft, USA) software was used to draw the various chemical structures of the ligand molecules.
Active Site Prediction: The Sitemap applies theoretical methods and predicts the most accurate binding site. A receptor grid was generated via the selection of the grid box. The binding site was recognized by specifying the atoms of a co-crystallized ligand (novobiocin). The scores were then calculated as the free energy of binding (ΔGb) and the final ten highest-scoring poses (conformations) for each molecule along with their scores and binding energies (ΔGb) were collated into a database. The database file generated from the docking procedure was further analyzed, with the binding mode (interactions) of the highest ten conformations for each docked molecule in the active site visualized and studied with the help of the Discovery studio visualization window
RESULT AND DISCUSSION
Chemistry: A series of various benzaldehydes substituted quinoxaline thiosemicarbazide derivatives (Va-Vf) were synthesized with a good percentage yield as per scheme Figure 1. Structure and physicochemical data of the final compounds represented in Table 1. The chemical structures of the compounds were confirmed by elemental analysis, IR, NMR & Mass spectroscopy.
The IR spectra of the compounds exhibit absorption bands due to OH, N-H, C-H, C=C, C=N, (CH (CH3)2, C=S and C-Cl stretching. The IR of synthesized compounds showed absorption bands near ranges 3215-3395 cm-1, 2932-3236cm-1 and 2839- 3097cm-1 correlated with N-H stretching, C-H sp2 aromatic stretching and C-H sp2 aliphatic stretching respectively. Also, the stretching absorption bands near ranges 1599-1668 cm-1, 1337-1398cm-1 and 1222-1272cm-1 correlated with C=N, -CH(CH3)2, and C=S groups respectively. IR displayed a characteristic broad absorption band at 3430 cm-1 for the OH group in compound Vd. The 1H-NMR at 300 MHz, the solvent used DMSO-d6 of all the derivatives showed a sharp singlet peak near range δ 1.27-1.95 ppm indicated isopropyl CH3 (6H) protons and δ 8.61-9.67 ppm indicated protons of Schiff bridge (N=CH). Multiple peaks appeared in all compounds ranges between δ 2.17-2.64 due to protons of isopropyl C-H. A broad set of singlet and doublet peak ranges δ 6.62-7.82 correlated to quinoxaline moiety aromatic hydrogens and sharp singlet peak range between δ10.40-11.28 indicated hydrogens of N-H group. A sharp singlet peak in compound Vd at δ 10.05 indicated hydroxyl group (OH) proton. The mass spectrum analysis of the compounds displayed characteristic peaks normally with [M+1]+ value and [M+2]+ value in compound Vd. The elemental analysis outcomes of the compounds almost ranged within ± 0.4% of the calculated values.
antimicrobial activity: The synthesized derivatives exhibited significant activity against Gram-negative and Gram-positive bacteria when compared with standard Ciprofloxacin antibacterial drug (Table 2). The compound Vb showed more potent activity against Gram-negative strains. aeruginosa (0.39 µg/ mL) and H. pylori (0.39µg / mL) and equipotent activity against K. pneumonia (0.19 µg/ mL), E. coli (0.19 µg / mL), and less active against S. Typhi (0.19 µg/ mL). The compound Vc exhibited good activity against H. pylori (0.39 µg / mL) but equipotent activity K. pneumoniae (0.19 µg/ mL), E. coli (0.19 µg / mL) and P. aeruginosa (0.78 µg/ mL). Whereas no compounds showed significant activity against S. typhi.
On the study of Gram-positive strains, a more excellent twofold activity was observed of compound Ve and Vf against B. subtilis (0.39 µg / mL), S. aureus (0.19 µg / mL) and MRSA (0.78 µg / mL) and equipotent activity against B. thuringiensis (0.39 µg / mL). The compound Vcalso showed twofold activity against B. subtilis (0.39 µg / mL) and equipotent activity against B. thuringiensis (0.39 µg / mL) and MRSA (1.56 µg / mL). Thus, it was found that compound B exhibited more potent activity against Gram-negative bacterial strains but less active against Gram-positive bacterial strains whereas compound Ve and Vf showed more potent activity against Gram-positive bacterial strains rather than Gram-negative bacterial strains. The study revealed that the compound Vc showed good activity against some Gram-negative strains as well as some Gram-positive strains when compared with reference standard drug ciprofloxacin.
The study of antifungal activity was tested against strains such as P. Chrysogenum, A. niger and C. Albicans using fluconazole as a standard drug (Table 2). Compound Vc and Vf exhibit a twofold amplified activity against A. niger(3.12 µg / mL) and C. Albicans (1.56 µg / mL), whereas equipotent activity against P. Chrysogenum(0.78 µg / mL).The compound Ve exhibit equipotent activity against. Chrysogenum(0.78 µg / mL) and A. niger(6.25 µg / mL), but less active against C. Albicans (6.25 µg / mL). The overall study revealed that compound Vc, Vfand some extent compound Veexplored the best potential activity against fungal strains in comparison with that of the standard compounds.
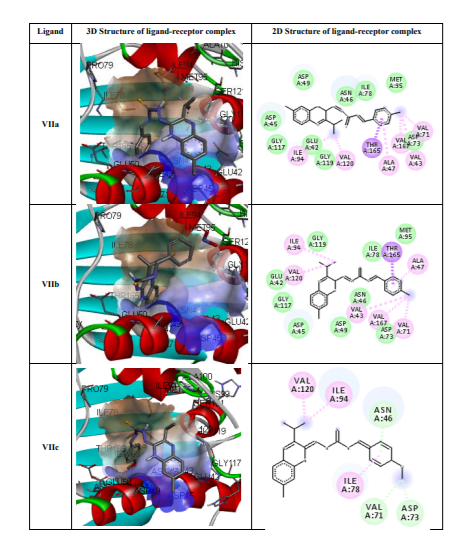
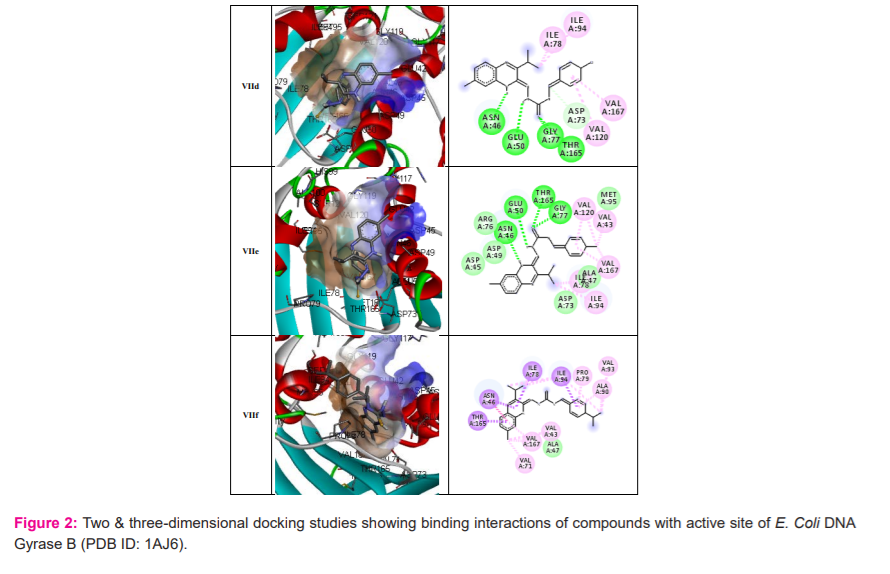
Molecular docking results: The docking of ligand molecules within the active pocket site of E. coli DNA gyrase B revealed that all the inhibitor compounds were exhibited the bonding with no. of amino acids which are showed in Figure 2. Theoretically, all the synthesized compounds showed very good docking energy ranging from -8.0 to -8.3 kcal/mol for PDB:1AJ6 and -7.6 to -8.2 for PDB:1S14 (Table 3).
We concentrated our attention on the more potent compounds Vb, Vc, Ve and Vfembedded nicely within the active pocket of E. coli DNA gyrase B (PDB: 1AJ6) with the binding energy of -8.0, -8.0, -8.3 and -8.1 Kcal/mol respectively. The interactions of the compounds revealed that the methyl groups of isopropyl present on the quinoxaline ring was involved in hydrophobic interaction with ILE94 and VAL20 that may explain the observation that isopropyl substitution on the quinoxaline ring enhances E. Coli DNA gyrase B inhibitory potency. The quinoxaline ring displayed hydrophobic interaction (arene-cation interaction) with ASN46 and THR165. Furthermore, the secondary amine group of the thiosemicarbazide moiety showed hydrogen linkage with GLU50 and thio group linked with THR165 and GLY177. The nitro and methoxy group in compound Vb and Vc showed binding with VAL43, VAL71 and VAL 167 (Figure 2).
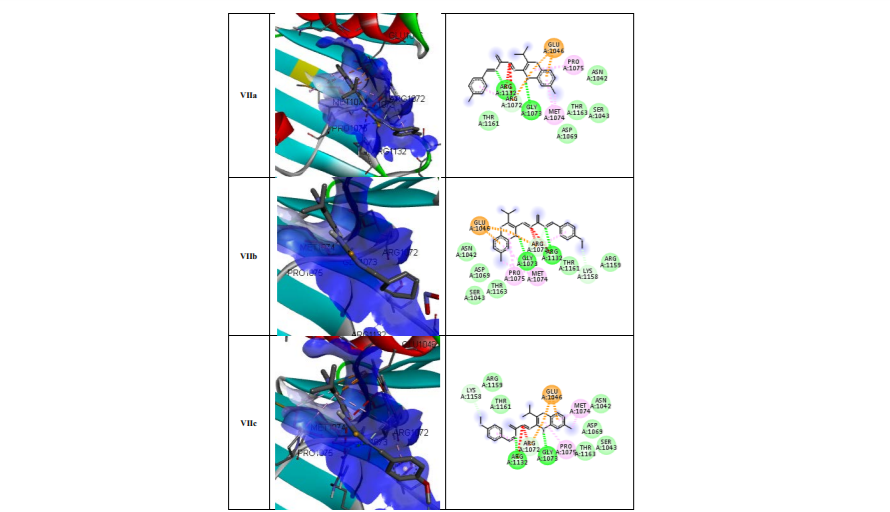
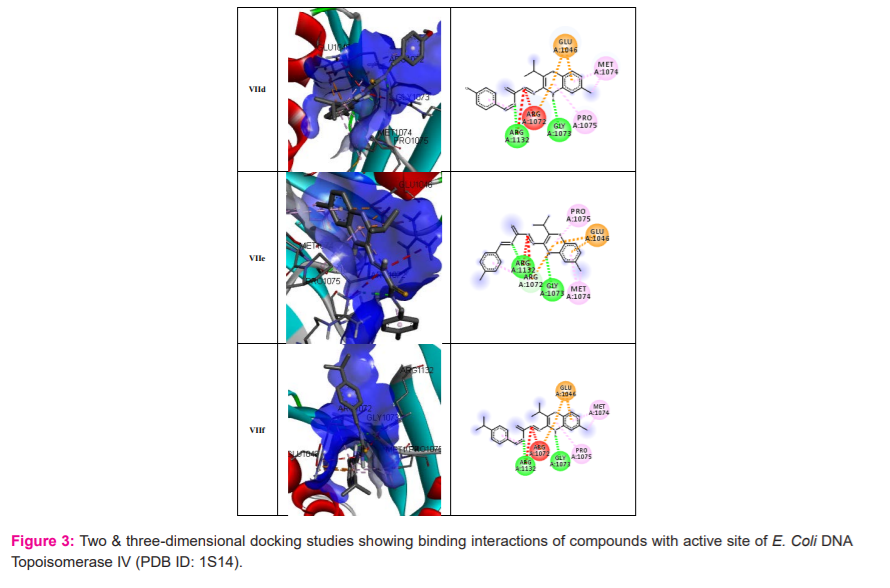
The docking study of the synthesized compounds against E. coli Topoisomerase IV using PDB code: 1S14 reveals that all the compounds exhibited good binding energy ranging from -7.6 to -8.2 kcal/mol (Table 4). The more potent compound Vb showed good docking energy (-8.2 kcal/mol) and docked effectively in the active pocket site of E. coli Topoisomerase IV. The ligand-protein complexes showed that the quinoxaline ring of each compound binds extensively through hydrophobic interactions with GLU1046 and MET1074. The NH of the quinoxaline ring displayed hydrogen bond linkage with GLY1073. The nitrogen of thiosemicarbazide moiety exhibit hydrogen and hydrophobic linkage with ARG1132 and ARG1072(Figure 3).
CONCLUSION
In conclusion, the present research reports the successful synthesis of different benzaldehydes substituted (1E, 4E)-1-(7-chloro-3-isopropyl quinoxaline- 2(1H)-ylidene) thiosemicarbazide derivatives and their in-vitro antimicrobial evaluation with molecular docking studies. Most of the evaluated compounds showed slightly more significant antimicrobial activity in comparison to reference compound. The structure-activity relationship study affirmed that the substitution by para-nitro benzaldehyde (Vb) enhance the activity against Gram-negative bacteria whereas substitution by para-methyl benzaldehyde (Ve) and para-isopropyl benzaldehyde (Vf) enhance the spectrum of activity against Gram-positive bacteria as well as fungal strains. Substitution by para-methoxy benzaldehyde as in compound Vc enhances the spectrum of activity against both Gram-negative as well as Gram-positive bacterial strains and also against fungal strains in comparison to reference compound.
The electron-withdrawing and donating groups substituted in the para position of benzaldehydes exhibited well binding with amino acids in the active pocket site of DNA gyrase B and E. coli Topoisomerase IV. Thus, following this research, the synthesized molecules could be considered as candidates for more clinically relevant researches in the future to overcome this type of antimicrobial resistance.
ACKNOWLEDGMENTS: The authors are thankful to the authorities, Department of Pharmacy, Bhagwant University Ajmer, Rajasthan, India and Goel institute of pharmacy and Sciences, Lucknow, U.P., India, for providing facilities to perform the research work and also thankful to the Department of Biotechnology of Saroj Institute of Technology & Management, Lucknow, India for providing bacterial and fungal strains.
AUTHORS’ CONTRIBUTIONS: Girendra Kumar Gautam: provided the concept and design of the study, acquisition of data, analysis and interpretation of data. Akash Ved: provided methodology of study and help in the drafting of the manuscript. Krishna Kumar Varshney: helped in molecular modelling study and interpretation of data. All authors read and approved the final manuscript.
HUMAN AND ANIMAL RIGHTS: No human and animals were used for studies in the present research work.
CONFLICT OF INTEREST: The authors declare no conflict of interest, financial or otherwise.
SOURCE OF FUNDING: TheAuthor received no specific funding for this research work.
References:
-
El-Sharief MAMS, Abbas SY, El-Bayouki KAM, E. W. El-Gammal EW. Synthesis of thiosemicarbazones derived from N-(4-hippuric acid)thiosemicarbazide and different carbonyl compounds as antimicrobial agents.Eur J Med Chem. 2013; 67:263–68.
-
Tenover FC.Mechanisms of antimicrobial resistance in bacteria. Am J Inf Cont. 2006; 34 Suppl 5:S3–S10.
-
Katritzky AR, Rees CW, editors. The structure, reactions, synthesis, and uses of heterocyclic compounds.Comprehensive Heterocyclic Chemistry. 1st ed. Oxford UK: Pergamon Press;1984:157–97.
-
Ganapaty S, Ramalingam P,Rao CB. Antibacterial, antifungal and antitubercular screening of some novel condensed bridgehead nitrogen heterocycles of quinoxalines. Ind J Hetero Chem. 2007; 16:283-86.
-
Refaat HM, Moneer AA, Khalil OM. Synthesis and antimicrobial activity of certain novel quinoxalines. Arch Phram Res. 2004; 27:1093-98.
-
Badran MM, Abouzid KAM, Hussein MHM. Synthesis of certain substituted quinoxalines as anti-microbial agents. Part II.Arch Pharm Res. 2003; 26:107–13.
-
Tandon VK, Yadav DB, Maurya HK, Chaturvedi AK,Shukla PK. Design, synthesis and biological evaluation of 1,2,3- trisubstituted-1,4- dihydrobenzo [g] quinoxaline-5,10-diones and related compounds as antifungal and antibacterial agents. Bioorg Med Chem. 2006; 14(17):6120-126.
-
Sanna P, Carta A, Loriga M, Zanetti S, Sechi L. Preparation and biological evaluation of 6/7-trifluoromethyl (nitro)-6,7-difluoro-3-alkyl(aryl)-substituted-quinoxalin-2-ones. Part III Farmaco. 1999; 54(3):1169-177.
-
Jaso A, Zarranz B, Aldana I, Monge A. Synthesis of new 2-acetyl and 2-benzoyl quinoxaline 1,4-di-N-oxide derivatives Asanti-mycobacterium tuberculosis agents. Eur J Med Chem. 2003; 38:791–800.
-
Rangisetty JB, Gupta CNVHB, Prasad AL, Srinivas P, Sridhar N, Parimoo P, et al. Synthesis of new-arylamino quinoxalines and their antimalarial activity in mice. J Pharm Pharmacol. 2001; 53(10):1409–13.
-
Crowther AF, Curd FHS, Davey DG, Stacey GJ. Synthetic antimalarials, Part 39 Dialkylaminoalkyl-aminoquinoxalines. J Chem Soc. 1949:1260–62.
-
Guillon J, Forfar I, Matsuda MM, Desplat V, Saliege M, Thiolat D. Synthesis, analytical behaviour and biological evaluation of new 4-substituted pyrrolo[1,2-a]quinoxalines as antileishmanial agents.Bioorg Med Chem Lett. 2007; 15:194-210.
-
Carta A, Sanna P, Gherardini, Usai D, Zanetti S. Novel functionalized pyrido[2,3-9]-quinoxalinones as antibacterial, antifungal and anticancer agents. II Farmaco. 2001; 56(12):933–38.
-
Sanna P, Carta A, Loriga M, Zanetti S, Sechi L. Synthesis of 3,6,7-substituted quinoxalin- 2-ones for evaluation of the antimicrobial and anticancer activity. Part: II Farmaco.1999; 54(3):161-68.
-
Hassan SY, Khattab SN, BekhitAA, Amer A. Synthesis of 3-benzyl-2-substituted quinoxalines as novel monoamine oxidase A inhibitors.Bioorg Med Chem Lett. 2006; 16(6):1753-56.
-
Sarges R, Howard HR, Browne RG, Lebel LA, Seymour PA, Koe BK. 4- Amino[1,2,4]triazolo[4,3-a]quinoxalines. A novel class of potent adenosine receptor antagonists and potential rapid-onset antidepressants. J Med Chem. 1990; 33(8):2240-54.
-
Burguete A, Pontiki E, Hadjipavlou-Litina VD.Synthesis and anti-inflammatory/antioxidant activities of some new ring-substituted 3-phenyl-1-(1,4-di-N-oxide quinoxalin-2-yl)-2-propen-1-one derivatives and of their 4,5-dihydro-(1H)-pyrazole analogues.Bioorg Med Chem Lett. 2007; 17(23): 6439-43.
-
Santivañez-Veliz M, Pérez-Silanes S, Torres E, Moreno-Viguri E. Hypoxia-Selective Agents Derived from Quinoxaline 1,4-Di-N-oxides. Bioorg Med Chem Lett. 2016; 26(9): 2188-93.
-
Ries UJ, Priekpe HW, Havel NH, Handschuh S, Mihm G, Stassen JM, et al. Heterocyclic thrombin inhibitors. Part 2: quinoxalinone derivatives as a novel, potent antithrombotic agents. Bioorg Med Chem Lett. 2003; 13:2297-02.
-
Palluotto F, Sosic A, Pinato O, Zoidis G, Catto M, SissiC, et al.Quinolino[3,4-b]quinoxalines and pyridazino[4,3-c]quinoline derivatives: Synthesis, inhibition of topoisomerase IIα, G-quadruplex binding and cytotoxic properties. Eur J Med Chem. 2016; 123:704-717.
-
Beraldo H, Gambino D. The wide pharmacological versatility of semi carbazones, thiosemicarba-zones and their metal complexes. Mini-Rev Med Chem. 2004;4(1):31–39.
-
Ramachandran R, Rani M, Kabilan S. Design, synthesis and biological evaluation of novel 2-[(2, 4-diaryl-3-azabicyclo [3.3. 1] nonan-9-ylidene) hydrazono]-1, 3-thiazolidine-4-ones as a new class of antimicrobial agents. Bioorg Med Chem Lett. 2009; 19(10): 2819–23.
-
Shipman Jr C, Smith SH, Drach JC, Klayman DL. Antiviral Activity of 2-Acetylpyridine Thiosemicarbazones Against Herpes Simplex Virus. Antimic Agen Chem. 1981; 19(4):682-85.
-
Quenelle DC, Keith KA, Kern ER. In-vitro and in-vivo evaluation of isatin-β-thiosemicarbazone and marboran against vaccinia and cowpox virus infections. Antiv Res. 2006;71(1):24–30.
-
Afrasiabi Z, Sinn E, Padhye S. Transition metal complexes of phenanthrenequinone thiosemicarbazone as potential anticancer agents: synthesis, structure, spectroscopy, electrochemistry and in-vitro anticancer activity against human breast cancer cell-line, T47D. J Inorg Biochem. 2003;95(4):306–14.
-
Singh N, Srivastava A, Sodhi A, Ranjan P. In- vitro and in- vivo antitumor studies of a new thiosemicarbazide derivative and its complexes with 3d-metal ions. Trans Met Chem 2000;25:133–40.
-
Yousef TA, Badria FA, Ghazy SE, El-Gammal OA, Abu El-Reash GM. In-vitro and in-vivo antitumor activity of some synthesized 4-(2-pyridyl)-3-Thiosemicarbazides derivatives. Inter J Med Sci. 2011;3(2):37–46.
-
Singh S, Bharti N, Naqvi F, Azam A. Synthesis, characterization and in-vitro antiamoebic activity of 5 nitrothiophene-2-carboxaldehyde thiosemicarbazones and their Palladium(II) and Ruthenium(II) complexes. Eur J Med Chem. 2004;39(5):459–65.
-
Sharma S, Athar F, Maurya MR, Naqvi F, Azam A. Novel bidentate complexes of Cu (II) derived from 5-nitrofuran-2-carboxaldehyde thiosemicarbazones with antiamoebic activity against E. histolytica. Eur J Med Chem. 2005;40(12):557–62.
-
Illan-Cabeza NA, Hueso-Urena F, Moreno-Carretero MN, MartinezMartos JM, Ramirez-Exposito MJ. Synthesis, Characterization and Antiproliferative Activity of Metal Complexes with the Schiff Base Derived from the Condensation 1:2 of 2,6-Diformyl-4- Methylphenol and 5,6-Diamino-1,3-Dimethyluracil. J InorgBiochem. 2008; 102:647–55.
-
Naveen VK, Vidyanand KR, Kirasur BN. Mallinath HH. Transition metal complexes of thiosemicarbazones with quinoxaline hub: an emphasis on antidiabetic property. Med Chem Res. 2012; 21(5):663–71.
-
Bal TR, AnandB, Yogeeswari P, Sriram D. Synthesis and evaluation of the anti-HIV activity of isatin beta-thiosemicarbazone derivatives. Bioorg Med Chem Lett. 2005; 15 (20):4451-55.
-
Ukrainets IV, Tkach AA, Grinevich LA, Turov AV, Bevz OV. 4-Hydroxy-2-quinolones 165.1-R-4-hydroxy-2-oxo-1,2-dihydro-quinoline-3-carbaldehydes and their thiosemicarbazones, Synthesis, structure, and biological properties. Chem Hetero Comp. 2009; 45:705–14.
-
Mohamed NA, Mohamed RR, Seoudi RS. Synthesis and characterization of some novel antimicrobial thiosemicarbazone O-carboxymethyl chitosan derivatives. Int J BioMacromol. 2014; 63:163–69.
-
Siddiqui N, Singh O. Antibacterial activity of some 4-N-substituted thiosemicarbazides and thiosemicarbazones. Ind J Pharm Sci. 2003; 65(4):423-25.
-
Parul N, Subhangkar N, Arun M. Antimicrobial activity of different thiosemicarbazone compounds against microbial pathogens. Int Res J Pharm. 2013; 3(5):351–63.
-
Siwek A, Stefanska J, Dzitko K, Ruszezak A. Antifungal effect of 4-arylthio semicarbazides against Candida species. Search for the molecular basis of antifungal activity of thiosemicarbazide derivatives. J Mol Model. 2012; 18(9):4159-70.
-
Naeem A, Badshah SL, Muska M, Ahmad N. Khan K. The Current Case of Quinolones: Synthetic Approaches and Antibacterial Activity. Molecules. 2016; 21(4):268.
-
Esteves-Souza A. Rodrigues-Santos CE, Del Cistia CN, Silva DR, Sant’anna CM, Echevarria A. Solvent-free synthesis, DNA-topoisomerase II activity and molecular docking study of new asymmetrically N, N’-substituted ureas. Molecules. 2012; 17: 12882–94.
-
Tanitame A, Oyamada Y, Ofuji K, Fujimoto M, Suzuki K, Ueda T, Terauchi H, Kawasaki M, et al. Synthesis and antibacterial activity of novel and potent DNA gyrase inhibitors with azole ring. Bioorg Med Chem. 2004; 12(21):5515–24.
-
Azimvand J. Synthesis of new triazole and oxadiazole containing compound in the azide reaction as an antibacterial drug.J Chem Pharma Res. 2012; 4(8):3900-04.
-
Efthimiou EK, Thomadaki H, Sanakis Y, Raptopoulou CP, Katsaros N, Scorilas A, et al. Structure and biological properties of the copper(II) complex with the quinolone antibacterial drug N-propyl-norfloxacin and 2,20 -bipyridine. J InorgBiochem. 2007; 101:64–73.
-
El Shehry MF, Ghorab MM, Abbas SY, Fayed EA, Shedid SA, Ammar YA. Quinoline derivatives bearing pyrazole moiety: Synthesis and biological evaluation as possible antibacterial and antifungal agents. Eur J Med Chem. 2017; 143:1463–73.
-
Paneth A, Staczek P, Plech T, Dzitko K, Wujec M, Kusmierz E, et al. Biological evaluation and molecular modelling study of thiosemicarbazide derivatives as bacterial type IIA topoisomerase inhibitors. J EnzymInhib Med Chem. 2016;31:14-22.
-
Siwek A, Staczek P, Wujec M, Stefanska J, Kosikowska U, Malm A, et al. Biological and docking studies of topoisomerase IV inhibition of thiosemicarbazides. J Mol Model.2011; 17:2297-03.
-
Mohi El-Deen EM, Abd El-Meguid EA, Hasabelnaby S, Karam EA, Nossier ES. Synthesis, docking studies and in vitro evaluation of some novel theinopyridines and fused theinopyridine-quinolines as antibacterial agents and DNA gyrase inhibitors. Molecules.2019; 24(20):36-50.
-
Bellon S, Parsons JD, Wei Y, Hayakawa K, Swenson LL, Charifson PS, et al. Crystal structure of E. coli topoisomerase IV ParE subunit (24 and 43 kilodalton): A single residue decades differences in novobiocin in potency against topoisomerase IV and DNA gyrase.Antimicrob Agents Chemot. 2004; 48(5):1856-64.
-
European Committee for antimicrobial susceptibility testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution. Clin Microb Infect. 2000; 6(9):509-515.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License