IJCRR - 13(13), July, 2021
Pages: 02-07
Date of Publication: 05-Jul-2021
Print Article
Download XML Download PDF
Nutrients Removal by Titanium Dioxide-Zeolite (TDZ) Composite
Author: Berhanuddin MS, Aris A, Chelliapan S, Abdul Majid Z, Hasaan HA
Category: Healthcare
Abstract:Introduction: One of the technologies used to remove nutrient is through zeolite and titanium dioxide (TiO2 ) composite. The photocatalytic process with TiO2 enables adopting this emerging water treatment technique as a safe alternative for the water contaminants removals due to its minimal energy costs and high-efficiency removal. Objective: The main objective of this study is to remove the Ammonia (NH4 +), Nitrate (NO3 - ) and Phosphate (PO4 +) nutrients from the wastewater sample using the inorganic adsorption process made with Titanium Dioxide-Zeolite (TDZ) composite. Methods: The mixtures were designed using the Design-Expert software (Version 7) (DX7) by studying the mixture types of D�optimal design. Two targeted samples of Ammonium Nitrate (NH4 NO3 ), and Ammonium Phosphate ((NH4 )H2 PO4 ) were diluted in one batch with the known Ammonia (NH4 +), Nitrate (NO3 - ) and Phosphate (PO4 +) concentrations. The composite of Titanium Dioxide (TiO2 )-Zeolite (TDZ) was developed using two types of adsorbent materials, i.e., natural zeolite and TiO2 and mixed with plaster cement. Results: The experiment concluded that the PO4 nutrient could be removed by the TDZ adsorbent with good adsorption, while the NH4 and NO3 nutrients are slightly satisfied in corresponding to the inorganic chemical process. Conclusion: The process is attributed to the ion exchangeability of TDZ, and it has the potential to be applied in removing the nutrients from treated wastewater.
Keywords: Nutrients removal, Titanium Dioxide, Zeolite, Composite, Environmental health
Full Text:
INTRODUCTION
In recent years, wastewater treatment using zeolite as a low-cost absorbent has been examined by many researchers. Zeolites, either natural or synthetic, can improve the water quality and enhance wastewater treatment by removing substances such as heavy metals, ammonium, phosphorus, dissolved organic matter, cations, and radioactive elements.1,2 Ion exchange based processes, such as using zeolites for the removals of ammonium from the wastewater, could be an attractive additional or potentially complementary treatment option. This is even more essential, especially for the treatment conditions that pose a challenge for biological processes, such as variable loads or low temperatures. Zeolite is a porous material, so both the external and internal (pores and channels) surface area can interact with the solution. By reducing the particle size, the external area increases considerably, but not to the internal area of the zeolite.3 It is considered the cheapest treatment method for removing nutrients from industrial/wastewater effluent.4 The phosphate removals process from aqueous solutions using natural Jordanian zeolitic tuff is a potentially viable and natural adsorbent material.5 Karap?nar also studied the application of natural zeolite for phosphorus and ammonium removals.5 Additionally, zeolite has been used in its modified form during the treatment process.
The application of nano-TiO2 as photocatalytic oxidiser has been well established. The materials’ semiconducting properties determine the performance of TiO2 for the conversion of solar energy into chemical energy. The conversion process is closely related to the light-induced reactivity between the oxide semiconductors and water, leading to partial water oxidation and, consequently, water disinfection.6 The various sizes of titanium dioxide (TiO2), such as bulk powder and nanoparticles, are used by varying the parameters, such as the pH or ionic strength of samples, to optimise the phosphorus removal from the wastewater. Gong et al. explored the effects of photocatalytic degradation in aqueous solution with a high concentration of ammonia, where immobilised TiO2 on glass beads was employed as the photocatalyst.7 In addition, Nano-TiO2 was also used as the coating for pet bottles used in keeping drinking water and for wastewater treatment. Moreover, it is also applied in stormwater quality improvement. Additionally, Lim et al. observed that TiO2 supported the activated carbon, or TiO2/AC composite, and exhibited bi-functionality of adsorption and photo-catalysis in synergism.8
The composite is defined as combining two or more materials that result in a compound with better application properties than the material produced with only one component. The main advantages of composite materials include their high strength and stiffness and low density compared with bulk materials, thus weight lesser for the finishing products. The removal of contaminants application by using dual-phase composite adsorbent is becoming more popular these days.9 The development of different composite types, ranging from nano-composites, activated charcoal composites, polymer composites, oxide-based composites, hybrid composites and biosorbent composites, has been reported.10 These composites are explored to treat or eliminate hazardous substances like heavy metal species, different classes of coloured contaminants (dyes), and several organic and inorganic pollutants from the wastewater.10 The D-optimal mixture design is widely used for the optimisation of the composite mixture. The function of this tool is to suggest the best mix ratio for the Titanium Dioxide-Zeolite (TDZ) components. The experiments are usually carried out according to the run number to minimise all the response values' variability.
This study is significant as it developed the nutrients removals technology from wastewater using inorganic process. The TDZ composite adsorbent developed in this study has satisfying adsorption performances and is economical, comprehensible and environmentally friendly. As the industry is always demanding low cost, lower discharge, environmentally friendly, readily available material usage, and the least space for the effluent treatment plant, most plants use the biological treatment as tertiary treatment. Moreover, biological treatment is vastly feasible and has more advantages than the other adsorption methods that are costly and difficult to be regenerated.
METHODOLOGY
Materials and chemicals
Ammonium nitrate (NH4NO3) and ammonium dihydrogen phosphate ((NH4)H2PO4) were supplied from Merck. Meanwhile, natural zeolite was supplied from NPK-Organo-Zeolite, TiO2 was purchased from Sigma-Aldrich, and the premixed plaster (921) cement was supplied from PYE (M) throughout the whole study. The HACH reagents used for the analysis of ammonia, nitrate and phosphate were purchased from Arachem (M) Sdn Bhd.
Analytical method
The analyses of NH4+, NO3- and PO4+ were carried out using the HACH DR 6000 (or DR3900) spectrophotometer. The surface and elemental composition of TDZ composite were identified using the Field-Emission Scanning Electron Microscopy (FESEM) (Hitachi, S-3400N model), coupled with an energy dispersive X-ray (EDX) analyser. Then, the surface composition and its distribution were determined by the EDX analyser.11 This FESEM model is a versatile analytical ultra-high resolution that extends the imaging and analytical resolutions beyond the previously achievable limits. Moreover, it has a unique variable pressure capability and enables the examination of various non-conducting samples without time-consuming preparation. Hence, the FESEM-EDX was performed to determine the elemental percentages, as well as to confirm the elemental distributions.12
Preparation of synthetic wastewater
The synthetic wastewater was prepared by dissolving 45.0 mg of NH4NO3 and 7.0 mg of (NH4)H2PO4 in 1000 mL distilled water (Systerm). This mixture produced ammonia, nitrate and phosphate concentrations of 10.0, 20.0, and 5.0 mg/L, respectively. These concentrations represented the Standard A of sewage effluent quality, as stipulated in the Environmental Quality Act 1974, published under the Environmental Quality (Sewage and Industrial Effluents) Regulations, 1999.13
Titanium dioxide-zeolite composite
The TDZ composite comprised of three main components, namely zeolite, TiO2 and premix plaster cement. These components were mixed together with water according to the ratio suggested by the Mixture experimental design. Each mixture was placed into a 1 cm3 mould and was cured for up to 7 days. The TDZ composite was then crushed into powder form with sizes of between 800µm to 500µm before used in the adsorption study. The best composition mixture of TDZ was identified by the adsorption experiment, followed by the analysis and optimisation using the D-optimal design.
Adsorption study
-
Mixture experimental design
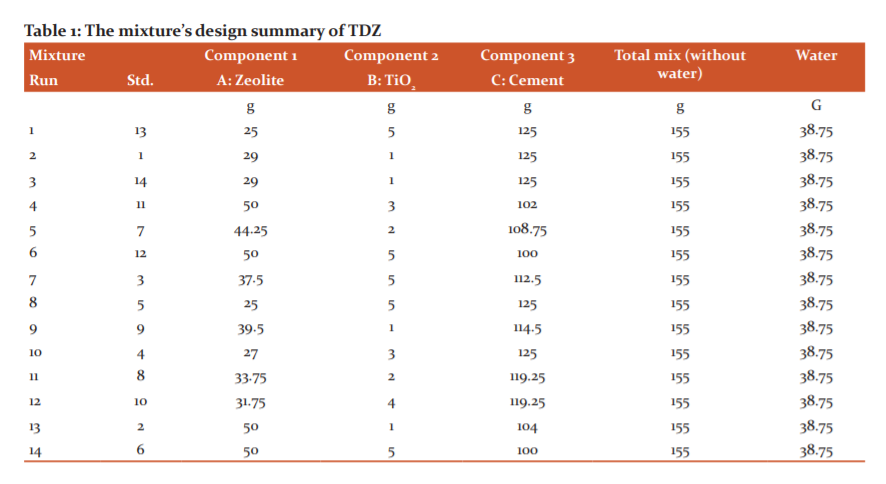
The mixture experimental design (D-optimal) was used to determine the optimum mixture ratio of the TDZ composite. The experimental design was developed using the Design Expert software (Version 7). As shown in Table 1, 14 experimental mixtures were tested. The component of zeolite, TiO2 and cement ranged from 25 to 50 g, 1 to 5 g, and 100 to 125 g, respectively. The ratios of TiO2:zeolite:cement was in the range between 5 to 50 to 100 and 1 to 25 to 125. The ratio between the mixture and water was set to 4 to 1. The performance of each TDZ composite mixture was evaluated based on the ability to remove ammonia, nitrate and phosphate.
The adsorption study was conducted in batch mode using a 100 mL conical flask. For each experimental run, a 100 ml aqueous solution with the known concentration of NH4, NO3 and PO4 was placed in the flask containing a 10 g of the TDZ composite adsorbent. These flasks were then shaken on an orbital shaker (Grant-Bio PSU-10i) at a constant shaking rate of 220 rpm to 240 rpm for 24 hours. After the shaking period ended, the supernatants were sampled, filtered with 0.45 µm filter and analysed for the contaminants.
RESULTS AND DISCUSSION
Characterisation of TDZ adsorbent
The TDZ composite image captured using the FESEM is shown in Figure 1. The analysis of the captured image revealed that the carrier material is uniform and grain sizes with variable coverage. Once focused, the working distance between the sample surface and the lower lens portion (WD = 10.1mm) of greater than 4 mm was measured. The utilization of magnification (mag = 200x) below 1000x showed significant detector artifacts. Therefore, the useful magnification with respects to the object analysis, line scan or mapping, was limited to 1000x. In addition, the high voltage (HV = 15 keV) of the highest peak, called overvoltage ratio, was determined to be not less than two for all the elements measured in this study.

Figure 2 shows the element compositions and the TDZ composite energies. The X-ray characteristics consist of the narrow emission lines, which are also the characteristic of the chemical elements contained in the sample. The energy of these lines was nearly independent of the chemical bonding state of the affected atoms, as the electron probe microanalysis is element sensitive. The results showed that the TDZ composite is composed of oxygen (O), calcium (Ca), titanium (Ti), silicon (Si), carbon (C), niobium (Nb) and aluminium (Al). These elements are acting as the adsorbents to remove the nutrients during the wastewater treatment.

TDZ mixture’s optimisation results
The 14 experimental runs for nutrients removals analysis are as shown in Figure 3. The tests were analysed by using the ANOVA statistic provided in the DX7 software. The removals of ammonia and nitrate ranged from about 22 to 70% and 10 to 18%, respectively. Meanwhile, the removal of phosphate was very high, ranging from about 95 to 99%. The level of significance was considered when P < 0.05. The ANOVA results for the measured responses (Figure 3) and the mixture Cubic Model analyses (Table 2) demonstrated that the model was significant for phosphate but not ammonia and nitrate.Based on Table 2, the significant model terms were shown to have real effects on the responses. The lack of fit reported indicated that the model does not fit the data within the observed replicate variation. The models were proceeded to the diagnostic plots and to optimise the mixtures.


Table 3 and Figure 4 describe the numerical and graphical optimisation, respectively, for the mixture of TDZ composite. There were six solutions recommended by the DX7 software, and the Number 1 mixture was selected as it exhibited the maximum desirability of 0.682. The Analysis of Variance (ANOVA) in DX 7 measured the R-Squared (R2) value. This value is a measure of the variation amount around the mean, as explained by the model. The R2 value for the PO4 adsorption was equal to 0.9933 and was better than the NH4 and NO3 adsorptions that recorded the R2 values of 0.7537 and 0.3478, respectively.


The desirability is defined as an objective function that ranges from zero to one at the goal. The desirability of 0.682 was selected in this study. The numerical optimisation calculated a point that maximises the desirability function. Based on the Design of Experiment (DoE) principle, the desirability range between 1.0 and 0.80 represents excellent and acceptable quality or performance. Meanwhile, the desirability range of between 0.80 to 0.63 describes the good and acceptable analysis, as well as representing a further improvement over the best commercial quality. The findings on the desirability study described the composite materials used and showed that the composite could contribute to remove three responses.
Water was used as the additional material in bonding the TDZ composite, with the ratio of 0.25 was used. Kovac et al. demonstrated that the changes in water to cement ratio from 0.25 to 0.35 caused only slight differences between the strength characteristics.14 Typically, the lesser water content in concrete mixture leads to the lesser porosity of cement paste. Thus, the desired mechanical properties are provided. In the case of conventional dense concrete, the lower water to cement ratio contributes to the higher or better strength, density and durability of concrete.
Figure 5 shows the three-D plot that is demonstrating the desirability of the TDZ adsorbent design. This graphical optimisation displays the feasible response values and areas in the factor space. The regions in the plot were fitted with the optimisation criteria. Furthermore, the performances of nutrients removals using TDZ composite were evaluated by using the adsorption isotherms and kinetic models.
CONCLUSIONS
The key findings emerged from this study revealed that the composite materials used were not duly significant in all the responses; for example, the nitrate and ammonia (p>0.05) materials showed insignificant results, but the phosphate (p<0.05) material showed the significant result. This observation suggests that the composite materials used to develop TDZ composite were reliable in removing the nutrients. The findings are important to understand the essential application of the selected TDZ composite to be used in the organic process of nutrients’ treatment. The process involves an inorganic chemical reaction with the elements of TiO2 and zeolite. The percentages of PO4 removal showed that the performance was good in the adsorption process, whereas the performance of NH4 removal was satisfied, while the NO3 removal showed very low action in adsorption.
Acknowledgment
The authors wish to thank Ministry of Higher Education Malaysia and Universiti Teknologi Malaysia for funding the publication fee for this study under the Vote Number: Q.K130000.2856.00L57. The authors also thank Universiti Kebangsaan Malaysia for providing resources, facilitate and support for this research.
Conflict of Interest: Nil
Source of Funding: Nil
References:
1. Deng Q. Ammonia removal and recovery from wastewater using natural zeolite: an integrated system for regeneration by air stripping followed ion exchange[dissertation]. University of Waterloo. 2014.
2. Kotoulas A, Agathou D, Triantaphyllidou IE, Tatoulis TI, Akratos CS, Tekerlekopoulou AG, et al. Zeolite as a potential medium for ammonium recovery and second cheese whey treatment. J Water. 2019; 11(1):136.
3. Yunnen C, Ye W, Chen L, Lin G, Jinxia N, Rushan R. Continuous fixed- bed column study and adsorption modeling: removal of arsenate and arsenite in aqueous solution by organic modified spent grains. Pol J Environ Stud. 2017;26(4):1847-1854.
4. Aljbour SH, Al-Harahsheh AM, Aliedeh MA, Al-Zboon K, Al- Harahsheh S. Phosphate removal from aqueous solutions by using natural Jordanian zeolitic tuff. Adsorpt Sci Technol. 2017; 35: 284-299.
5. Karap?nar N. Application of natural zeolite for phosphorus and ammonium removal from aqueous solutions. J Hazard Mater. 2009; 170: 1186-1191.
6. Wu MJ, Bak T, O’Doherty PJ, Moffitt MC, Nowotny J, Bailey TD, et al. Photocatalysis of titanium dioxide for water disinfection: Challenges and future perspectives. Int. J Photochem. 2014; 973484.
7. Gong X, Wang H, Yang C, Li Q, Chen X, Hu J. Photocatalytic degradation of high ammonia concentration wastewater by TiO2. Future Cities Envt. 2015; 1: 12.
8. Abdollahii S, Faezeh J , Marjan S , Amir MA. Adverse effects of some of the most widely used metal nanoparticles on the reproductive system. J Infert Reprod Bio. 2020, 8: 22-32.
9. Moideen SNF, Din MFM, Rezania S, Ponraj M, Rahman AA, Pei LW, et al. Dual phase role of composite adsorbents made from cockleshell and natural zeolite in treating river water. J King Saud Univ Sci. 2020; 32: 1- 9
10. Jaspal D, Malviya A. Composites for wastewater purification: A review. Chemosph. 2020; 246: 125788.
11. Aziz, MHA, Othman, MHD, Alias, NA, Nakayama, T, Shingaya, Y, Hashim, NA, et al. Enhanced omniphobicity of mullite hollow fiber membrane with organosilane- functionalized TiO2 micro-flowers and nanorods layer deposition for desalination using direct contact membrane distillation. J Membr Sci. 2020; 607:118137.
12. Li N, Jayaraman, S, Tee, SY, Kumar, PS, Lee, CJJ, Liew, SL, Chi, D, et al. Effect of La-Doping on optical bandgap and photo electrochemical performance of hematite nanostructures. JMater. Chem. A 2014; 2:19290.
13. Sabeena AH, Ngadia N, Noor ZZ, Raheema AB, Agouillal F, Mohammed AA, et al. characteristics of the effluent wastewater in sewage treatment plants of malaysian urban areas. Chem Eng Trans. 2018; 63:691 – 696.
14. Kovac M, Sicakova A. Changes of strength characteristics of pervious concrete due to variations in water to cement ratio. IOP Conf. Earth Environ Sci. 2017; 92: 012029
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License