IJCRR - 13(12), June, 2021
Pages: 128-132
Date of Publication: 22-Jun-2021
Print Article
Download XML Download PDF
Protocol on Assessment of Maternal and Fetal Outcome by Using Antenatal Mother's Overview List (Anmol) Mobile Application
Author: Kavita J. Gomase, Vaishali Taksande
Category: Healthcare
Abstract:Background: Most of the women die during pregnancy and following childbirth which results due to complications in pregnancy. These complications develop during pregnancy and most are preventable or treatable. Most of the complications remain unassessed. Severe morbidity during pregnancy has been overlooked, and prevention has been neglected despite its vital importance in improving outcomes. Mother complications may exist before or during pregnancy but are worsened during the peripartum period, especially if not managed as part of the antenatal care or not detected early in labour. Aim: To evaluate the effectiveness of Antenatal Mother's Overview List (ANMOL) Mobile Application in terms of Maternal And�Fetal Outcome as compare to contemporary protocol. Methods: This paper describes a randomized controlled trial, which aims to recruit 300 participants in this trial study. In each group, 150 women who are in labour (>4 cm cervical dilatation) will be selected for the study. The study will be conducted in the Labour room of the department of Obstetrics and Gynaecology. The first group (experimental group) will be assessed maternal and fetal outcome by using ANMOL mobile application. And second group (control group) will be assessed maternal and fetal outcome by following contemporary protocol. Then the comparison will be done among two groups in terms of maternal and fetal outcome. Ethics approval was obtained from IEC, DMIMS (DMIMS(DU)/IEC/2018-19/7131). The conclusion will be drawn from the results and will be published in a peer-reviewed journal. Results: This study is registered with the clinical trial registry- India. (Registration No. CTRI/2020/07/026846) The trial will be conducted in eight months. Recruitment started in August 2020 will continue until March 2021. Conclusion: Once ANMOL mobile application utilization is validated, these can be introduced in other remote area or low eco�nomic resources with a lack of trained manpower and facilities. This mobile application can be utilized without an internet facility
Keywords: Maternal severe complications during delivery, Mode of delivery, maternal death, APGAR score, stillbirth and early neo�natal death, Fetal
Full Text:
INTRODUCTION:
One of the great wonders of nature is the growth of a foetus within its mother. The growth and development of the baby are dependent upon the health and nutrition of the mother (not the father) because she is both the seed as well as the soil where the baby is nurtured for nine months. Pregnancy and childbirth are special events in women’s lives and indeed, in the lives of their families. This can be a time of great joy and joyful anticipation. It can also be a time of fear, suffering and even death. Even though pregnancy is not a disease; but a normal physiological process, it is associated with certain risks to the health and survival both for women and fetuses.1
Maternal death and disability are the leading causes of healthy life years lost for developing country women of reproductive age, accounting for more than 28 million disability-adjusted life years (DALYS) lost and at least 18% of the problem of disease in these women. Most women survive in childbearing but who was suffering from serious disease, disability or physical damage caused by pregnancy-related complications.2
Maternal mortality is a neglected tragedy and it has been neglected because those who suffer are neglected people with the least power and influence, they are the poor, the rural peasants and above all women.3Globally, levels of maternal mortality have remained stable since 1995. About 510,000 maternal deaths occurred worldwide during the year 2002.4 In addition to maternal mortality, seven million more women suffer serious health problems related to childbearing, and 50 million suffer adverse health effects.5Maternal morbidity and maternal deaths are a significant cause of death in women in the 15-49 years age group, and they make up a larger proportion of all-cause deaths in the rural areas of poorer states, compared to other regions of India.6
Definition: The maternal mortality rate (MMR) is the annual number of female deaths per 100,000 live births from any cause related to or aggravated by the pregnancy or its management (excluding accidental or incidental causes). The MMR includes deaths during pregnancy, childbirth, or within 42 days of termination of pregnancy, irrespective of the duration and site of the pregnancy, for a specified year.7
Pregnancy and labour are physiological events and should have a joyful culmination with a healthy mother and a healthy baby, however, the potential for dramatic and even catastrophic complications during pregnancy, labour or postpartum are real, either because of aggravation of a pre-existing illness or disorder arising during pregnancy, labour, postpartum. It is estimated that maximum maternal deaths could be prevented or avoided through actions that are proven to be effective and affordable, and none of the interventions is complex or beyond the capacity of a functional health system even in resource-poor countries. The number of deprived is likely to be much higher in India because of its vast population and diversity in the literacy levels and health facilities available. Non-availability of accessible, acceptable, quality health care, including emergency obstetric care during pregnancy and childbirth, compounded by the inability of women to recognize the need and seek health care during pregnancy and childbirth is the cause of high morbidity rates in developing countries. Swanton et al. have reported that diastolic blood pressure was included in all 9 obstetric specific EWS that they reviewed. Goldhill et al. noted the most common abnormalities to be tachypnoea and altered level of consciousness in patients admitted to ICU.8
In published literature by Singh et al. 2012, the MEOWS chart in the UK population is 89% sensitive, 79% specific with a positive and negative predictive value of 39% and 98% respectively. Though the results of our study 86.4% sensitive and 85.2% specific are comparable to the study by Singh et al. the few minor differences could be explained by the difference in the prevalence of obstetric morbidity for the Indian subcontinent9.
Baskett et al. have reported that delay in seeking care and transfer is one of the main factors leading to morbidity10.
Baja et al. in Banur, India also found poor transport facility, poor rural health infrastructure, custom and traditions to be contributing factors towards increase morbidity.11
The major complications during labour are severe bleeding, infection, high blood pressure, unsafe abortion. While admitting women in the labour room for safe confinement, she ensures that her delivery should be done safely without any complications. Earlier there is the protocol of making the written formats for her all assessment which is starting from the admission. While assessing the patient there was an inability to focus most of the parameters. As the labour process is the crucial events, the timely decision is very much important.
RATIONALE OF STUDY:
Deterioration of maternal health can occur at a very rapid rate, with catastrophic consequences therefore early recognition is essential. ANMOL mobile application is a simple objective useful tool to record and aid in recognition of maternal morbidity at an early stage, ultimately halting the cascade of severe maternal morbidity and mortality. By doing this study we will be able to test the effectiveness of the Antenatal Mother Overview List mobile application to enhance the maternal and fetal outcome time before the patient goes into critical condition.
AIM OF THE STUDY:
The aim of this study to evaluate the effectiveness of the Antenatal Mother’s Overview List (ANMOL) Mobile Application in terms of Maternal AndFetal Outcome as compare to contemporary protocol.
METHODOLOGY:
This study will be based on an experimental approach. The study will be conducted in the Labour room of the department of Obstetrics and Gynaecology of AVBRH, Sawangi.
Inclusion Criteria
Exclusion Criteria
-
Pregnant Women With High-Risk Pregnancies (Pregnancy Induced Hypertension, Gestational Diabetes Mellitus, Heart Diseases, Renal Diseases, IUGR)
-
Pregnant Women with Preterm Labor.
Sample size:
Sample Size Formula: N = X2 .N .P(1-P)/
C2(N-1) + X2.P(1-P)
Total number of deliveries in last year in AVBRH = 1288
Sample Size= 3.84 x1288 x 0.5 x 0.5
(0.05)2 x 1287+ 3.84 x 0.5x0.5
= 295.98 = 300
Total number of patients to be surveyed = 300
Randomization – All the participants will be assigned simple randomly by the coin toss method. Method of Concealment will used sequentially numbered, sealed, opaque envelopes.
Blinding- Participant Blinded
Interventions- ANMOL mobile application the tools used for assessing the physiological parameters in terms of maternal and foetal outcome. For collecting data in ANMOL mobile application, the women admitted in the labour room with >4 cm cervical dilation and with more than 34 weeks gestation will be enrolled for the using ANMOL mobile application. Control group which follows the contemporary protocol for assessing maternal and foetal outcome.
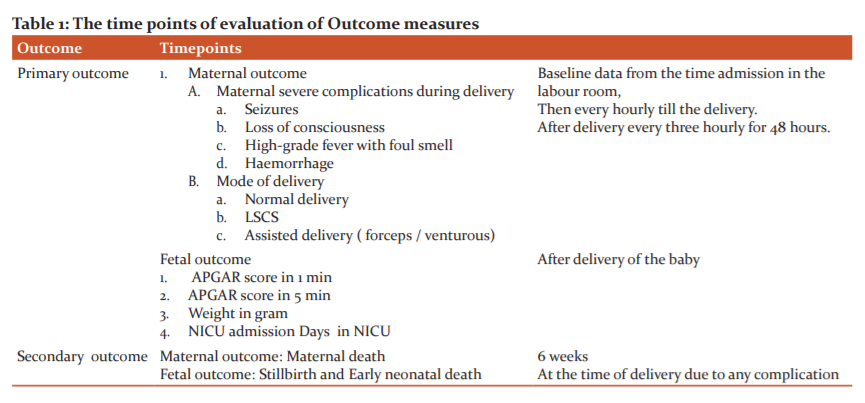
Outcome measures
For each pregnancy, the outcome was dichotomized as 'good' or 'adverse'. The outcome of pregnancy was coded as adverse if there was either an adverse maternal outcome or an adverse foetal outcome.
The outcomes of pregnancy studied are:-
1.Maternal outcomes
- Mortality - Maternal death
- Morbidity - Abnormal type of delivery-i.e., Caesarean section, forceps delivery, vacuum delivery, postpartum haemorrhage, puerperal pyrexia, seizures, shock.
2. Neonatal outcomes –
- Mortality Perinatal mortality, stillbirth and neonatal death.
- Morbidity. Birth injuries, neonatal infection, neonatal jaundice, respiratory distress, hypothermia, hypoglycemia,
The time points of evaluation are shown in Table 1
Data management and monitoring:
In ANMOL mobile application, Demographic variable includes followings physiological parameters-Patient ID, Name, Age, Hemoglobin, Edema, Hb electrophoresis, RBS, Platelets, Delivery details, Birth status, Type of delivery, Bleeding amount, Gender of baby, Weight of baby, APGAR Score, DOB & TIME, Fetal Heart rate.
In ANMOL– 1. The patient will be assessed with the following physiological parameters at the interval of one hour till the 24 h after delivery.
It includes respiration rate, saturation, temperature, heart rate, systolic blood pressure, diastolic blood pressure, urine output, proteinuria, amniotic fluid, neuro, pain score, looks, SPO2.
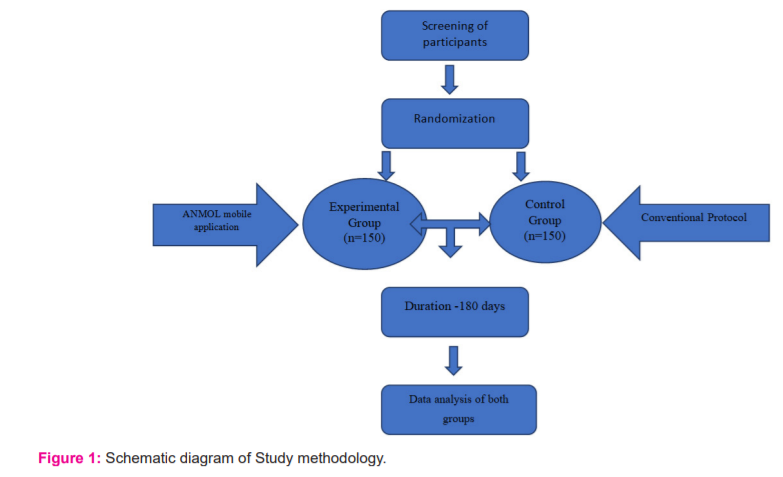
The application is based on the principle that abnormalities in physiological parameters precede a critical illness. The 'track and trigger' of physiological parameters on this chart can aid in the recognition of maternal morbidity at an early stage, ultimately halting the cascade of severe maternal morbidity and mortality. A trigger is defined as a single markedly abnormal observation (red trigger) or the combination of two simultaneously mildly abnormal observation (two yellow triggers). The second group will be the control group which follows the contemporary protocol for the maternal and fetal outcome of labouring women. Step 3 -For collecting data, women admitted in the labour room with >4 cm cervical dilation and with more than 34 weeks gestation will be enrolled for the study in the control group. A schematic diagram of the Study methodology is shown in Figure no. 1.
Statistical analysis-
Statistical analyses will be performed using SPSS software version 22. Paired t-test (Wilcoxon sign rank) and unpaired t-test (Wilcoxon Rank-sum) will be applied to analyze the data.
Ethics and dissemination:
This study is approved by the Institutional Ethics Committee of DMIMS (DMIMS (DU)/IEC/2018-19/7131). All participants will be asked to read and sign the informed consent. The study results will be disseminated to study participants and published in peer-reviewed publications.
RESULTS
Once the ANMOL mobile application utilization is validated, these can be introduced in other remote area or low economic resources with a lack of trained manpower and facilities. This mobile application can be utilized without an internet facility. The findings of this study will have implications on obstetrics unit to recognize deviation from normal monitoring of labouring women and enhance the labour outcome by ANMOL mobile application.
CONCLUSION:
A conclusion will be drawn from the statistical analysis.
CONFLICTS OF INTEREST: Nil
FINANCIAL SUPPORT: Self
ACKNOWLEDGEMENT:
I would like to express my sincere thanks to all faculties of Smt. Radhikabai nursing College, Sawangi (Meghe) Wardha, India for smooth completion of my research work. We acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. We would like to thank the authors whose works have cited and included in this study such as Singh S, McGlennan A, England A, Simons R T.Baskett et al, Bajwa et al.We are also grateful to the authors/editors/publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
-
-
-
Hillman KM, Bristow PJ, Chey T, Daffurn K, Jacques T, Norman SL, Bishop GF, Simmons G. et al. Duration of life-threatening antecedents before intensive care admission. Int Care Med. 2002 Nov;28(11):1629-34.
-
Bowyer L. The confidential enquiry into maternal and Child health (CEMACH). Saving mothers’ lives: reviewing maternal deaths to make motherhood safer 2003–2005. The seventh report of the confidential enquiries into maternal deaths in the UK.
-
Program NH. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am J Obst Gynec. 2000 Jul 1;183(1):s1-22.
-
Say L, Souza JP, Pattinson RC, WHO working group on Maternal Mortality and Morbidity classifications. Maternal near-miss—towards a standard tool for monitoring the quality of maternal health care. Clin Obstet Gynaecol. 2009;23(3):287–96.
-
Morgan RJ, Williams F, Wright MM. An early warning scoring system for detecting developing critical illness. Clin Intensive Care. 1997;8(2):100.
-
Friedman AM. Maternal early warning systems. Obstet Gynecol Clin North Ame. 2015;42(June (2)):289–98.
-
Mhyre JM, D'Oria R, Hameed AB, Lappen JR, Holley SL, Hunter SK, Jones RL, King JC, D'Alton ME. The maternal early warning criteria: a proposal from the national partnership for maternal safety. J Obst, Gynec Neon Nur. 2014 Nov 1;43(6):771-9
-
Quinn AC, Meek T, Waldmann C. Obstetric early warning systems to prevent a bad outcome. Curr Opin Anesth. 2016 Jun 1;29(3):268-72.
-
Singh S, McGlennan A, England A, Simons R. A validation study of the CEMACH recommended modified early obstetric warning system (MEOWS). Anae 2012;67(1):12.8
-
Baskett T, Connell M. Maternal critical care in obstetrics. J Obstet Gynaec. 2009;31:218–21.
-
Bajwa SK, Bajwa SJ, Kaur J, Singh K, Kaur J. Is intensive care the only answer for high-risk pregnancies in developing nations? J Emerg Trauma Shock. 2010;3:331–6.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License