IJCRR - 13(12), June, 2021
Pages: 88-93
Date of Publication: 22-Jun-2021
Print Article
Download XML Download PDF
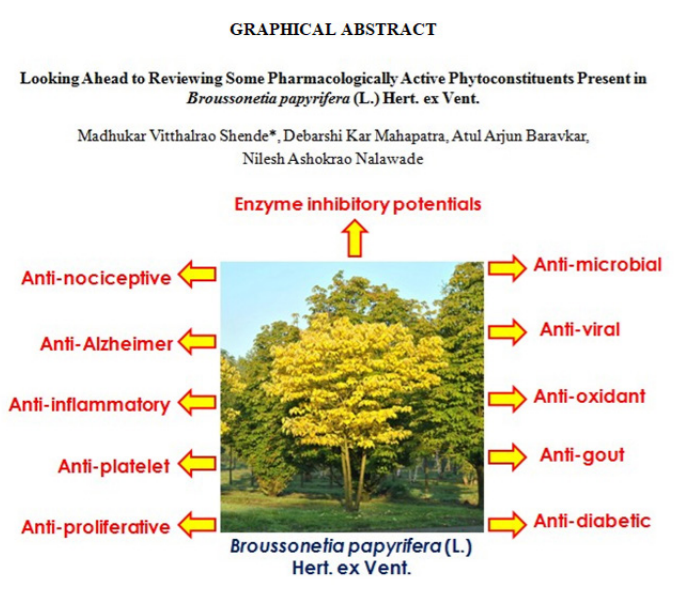
Looking Ahead to Reviewing Some Pharmacologically Active Phytoconstituents Present in Broussonetia papyrifera (L.) Hert. ex Vent.
Author: Madhukar Vitthalrao Shende, Debarshi Kar Mahapatra, Atul Arjun Baravkar, Nilesh Ashokrao Nalawade
Category: Healthcare
Abstract:Introduction: Plants have long been used to cure diverse ailments and disorders as a source of conventional medicines. Many of these medicinal plants are also fantastic phytochemical sources, many of which have strong therapeutic practices. Broussonetia papyrifera, also known as paper mulberry, is a well-known traditional natural resource that has been in application for decades and the renowned advances must be presented to researchers for further betterment, product innovation, exploring novel applications, and uncover miscellaneous ideas. Aim: Reviewing some pharmacologically active phytoconstituents present in B. papyrifera Linn. Methodology: The systematic literature compilation about the basic aspects, distribution, plant profile, pharmacological advances, key plant parts, ethnopharmacology, and other crucial information about B. papyrifera was performed through freely available scientific databases / natural products databases such as ScienceDirect, Google Scholar, PubMed, etc. Results: This current fascinating article expansively emphasized the general aspects, plant profile (Kingdom, Sub-Kingdom, Infra-Kingdom, Division, Sub-Division, Super-Division Class, Order, Super-Order, Family, Genus, and Species), traditional uses, distribution, major phytoconstituents, significant pharmacotherapeutic attributes (anti-viral, anti-cancer, anti-oxidant, cytotoxic potentials, anti-inflammatory, anti-diabetic, anti-microbial, anti-nociceptive, anti-gout, and anti-proliferative) mediated by diverse parts (seed, root, leaf, stem, and fruit). Conclusion: This information will be reasonably functional for the passionate contemporary investigators of several areas (natural products, pharmacognosy, medicine, chemistry, botany, pharmacy, etc.) in developing miscellaneous essential formulations for treating numerous disorders such as inflammation, cancer, high blood sugar, pain, infection, along with exhibiting cellular protective effects. This study will pave a new way for modern nature-pharmacotherapeutics for human applications
Keywords: Broussonetia papyrifera, Paper mulberry, Phytoconstituents, Ethnopharmacology, Therapeutics, Traditiona
Full Text:
INTRODUCTION
Plants have long been used to cure diverse ailments and disorders as a source of conventional medicines. Many of these medicinal plants are also fantastic phytochemical sources, many of which have strong therapeutic practices. The genus Broussonetia was named after P.N.V. Broussonet, a French naturalist, who took a male tree of B. papyrifera from a garden in Scotland, and introduced it to Paris, France, where a female tree was growing, thus enabling fruit to be described.1 The genus contains 8 species, of which 7 are native to Asia and one to Madagascar. There are 16 or 17 recognized varieties of the East Asian species, including 5 wild varieties. The specific name papyrifera means paper-bearing. The paper made from wild varieties is inferior to that from non-wild varieties.2
B. papyrifera (L.) L'Her. ex Vent. (Paper mulberry) is a fast-growing shade tree belonging to the Moraceae family that is widely distributed throughout East Asia.3 It is cultivated within its natural range for its bark. It is native to China, Taiwan, Korea, and Japan and possibly native to the Pacific islands of Hawaii and Samoa. From India and Pakistan to Thailand, Malaysia, and the Pacific Islands, and even in North America, it has been naturalized in Asia. It is now widely found from sea level to 1000 m altitude in several locations in India and Pakistan.4
TAXONOMY
-
Kingdom: Plantae
-
Sub-Kingdom: Virdiplantae
-
Infra-Kingdom: Streptophyta
-
Super-Division: Embryophyta
-
Division: Tracheophyta
-
Sub-Division: Spermatophytina
-
Class: Magnoliopsida
-
Super-Order: Rosanae
-
Order: Rosales
-
Family: Moraceae
-
Genus: Broussonetia
-
Species: papyrifera
TRADITIONAL USES / ETHNOPHARMACOLOGY
B. papyrifera (Moraceae), also known as paper mulberry, grows naturally in Asia and Pacific countries. Its dried fruits have been used as a traditional Chinese medicine for the treatment of ophthalmic disorders and impotency.5 The leaves, twig roots, and barks of this plant are widely used to treat gynecological bleeding, dropsy, dysentery diseases as a folk medicine in China.6 The dried branches, leaves, and roots of this plant are used as a Korean traditional medicine for various therapeutic purposes, such as a diuretic, tonic, and suppressor of oedema.7,8 In particular, isolated metabolites from the roots have multiple biological characteristics including anti-inflammatory,9 anti-asthmatic,10 anti-oxidant,11 anti-cancer,12 anti-nociceptive,13 anti-microbial,14 PTP-1B inhibition,15 and aromatase enzyme inhibition.16 The extracts of this plant have also been described by the Korea Food and Drug Administration (KFDA) as a medicinal ingredient of Korean traditional medicine, and its effectiveness has been supported by the recent identification of bioactive metabolites, including chalcones, flavonoids, and flavonols with potential therapeutic activities like anti-cancer,12 α-glucosidase,7 anti-cholinesterase,17 xanthine oxidase,11 and anti-platelet activities.18
PHYTOCHEMISTRY
Phytochemicals reported in B. papyrifera are: broussonin A; broussonin B; (+)-marmesin; kazinol F; broussochalcone A; 1-(2,4-dihydroxyphenyl)-3-(4-hydroxyphenyl)-propane; 1-(4-hydroxy-2-methoxyphenyl)-3-(4-hydroxy-3-prenylphenyl)-propane; 3’-γ-hydroxymethyl-(E)- γ-methylallyl-2,4,2’,4’-tetrahydroxychalcone-11’-O-coumarate; (2S)-2’,4’-dihydroxy-2”-(1-hydroxy-1-methylethyl)dihydrofuro-2,3-h flavanone; isolicoflavonol; (2S)-abyssinone II; (2S)-5,7,2’,4’-tetrahydroxyflavanone; (2S)-euchrenone a7; broussoflavonol F; (2S)-naringenin (Syn. Naringetol); albanol A (Syn. Mulberrofuran G); moracin N; isogemichalcone C; chushizisin H; broussoflavonol E; broussoflavonol G; broussoflavonol C; broussoflavonol D; chushizisin I; 5,7,3’,4’-tetrahydroxy-3-methoxy-6-geranylflavone; broussoflavonol B; broussoflavonol A; 5,7,3’,4’-tetrahydroxy-6-geranylflavonol; 4’-O-methyldavidioside; broussoflavan A; (2R,3R)-lespedezaflavanone C; broussoflavonol F; 5,7,2’,4’-tetrahydroxy-3-geranylflavone; kazinol A; kazinol B; gancaonin P; uralenol; (2S)-2’,4’-dihydroxy-2”-(1-hydroxy-1-methylethyl)dihydrofuro-2,3-h flavanone; isolicoflavonol; chushizisin C; chushizisin D; chushizisin E; chushizisin B; chushizisin A; chushizisin F; (2S)-euchrenone a7; broussochalcone A; broussoaurone A; chushizisin G; broussinol; isobavachalcone; broussochalcone B; (2S)-abyssinone II; bavachin; moracin I; broussonin C; (2S)-7,4’-dihydroxy-3’prenylflavan; moracin N; demethylmoracin I; moracin D; broussonin F; broussin; 7,4’-dihydroxyflavan; pinocembrin; resveratrol; isoliquiritigenin; isoliquiritigenin 2’-methy ether; 2,4,2’,4’-tetrahydroxychalcone; (+)-dihydrokaempferol (Syn. (+)-aromadendrin); norartocarpanone (Syn. Steppogenin); dimethoxy isogemichalcone C; moracin M; (2S)-7,4’-dihydroxyflavan; broussonin E; 1,2,4-dihydroxy-3-(3-methylbut-2-en-1-yl)phenyl-3-(2,4-dihydroxyphenyl)-propan-1-one; 2-{5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-3,4-dihydro-2Hchromen-8-ylamino}pentanedioic acid; papyriflavonol A (Syn. Broussonol E); (2R,3R,5R,6S,9R)-3-hydroxy-5,6-epoxy-β-ionol-2-O-β-D-glucopyra-noside; (2R,3R,5R,6S,9R)-3-hydroxyl-5,6-epoxy-acety-β-ionol-2-O-β-D-glucopyranoside; quercetin (Syn. 3,3’,4’,5,7-pentahydroxyflavone); 5,7,3’,5’-tetrahydroxyflavanone; luteolin; 5,7,3’,4’-tetrahydroxy-3-methoxyflavone; squalene; octacosan-1-ol; lignoceric acid; 4’-hydroxy-cis-cinnamic acid octacosyl ester; (–)-marmesin; 8-(1,1-dimethylallyl)-5’-(3-methylbut-2-enyl)-3’,4’,5,7-tetrahydroxyflanvonol; 3’-(3-methylbut-2-enyl)-3’,4’,7-trihydroxyflavane; kazinol E; sesquineolignan; 2-(4-hydroxyphenyl)propane-1,3-diol-1-O-β-D-glucopyranoside; 4-hydroxybenzal-dehyde; protocatechuic acid; broussonpapyrine; nitidine; oxyavicine; liriodenine; cosmosiin; (+)-pinoresinol-4’-O-β-D-glucopyranosyl-4”-O-β-D-apiofuranoside; luteolin-7-O-β-D-glucopyranoside; liriodendrin; 3,5,4’-trihydroxy-bibenzyl-3-O-β-D-glucoside; apigenin-6-C-β-Dglycopyranside; 8,11-octadecadienic acid;, broussoside A; broussoside B; broussoside C; broussoside D; broussoside E; syringaresinol-4’-O-β-D-glucoside; p-coumaric acid; apigenin; poliothyrsoside; pinoresinol-4’-O-β-D-glucopyranoside; flacourtin; dihydrosyringin; apigenin-7-O-β-D-glucoside; chrysoriol-7-O-β-D-glucoside; isovitexin; luteoloside; orientin; vitexin; isoorientin; 3,4-dihydroxyisolonchocarpin; 4-hydroxyisolonchocarpin; 3’-(3-methylbut-2-enyl)-3’,4’,7-trihydroxyflavane; 8-(1,1-dimethylallyl)-5’-(3-methylbut-2-enyl)-3’,4’,5,7-tetra-hydroxyflanvonol; broussofluorenone A; brusso fluorenone B; threo-1-(4-hydroxy-3-methoxyphenyl)-2-{4-(E)-3-hydroxy-1-propenyl-2-methoxyphenoxy}-1,3-propanediol; arbutine; dihydro-coniferyl alcohol; coniferyl alcohol; ferulic acid; p-coumaraldehyde; cis-syringin; cis-coniferin; erythro-1-(4-hydroxyphenyl)glycerol; threo-1-(4-hydroxyphenyl)glycerol; curculigoside I, curculigoside C, (2S)-2’,4’-dihydroxy-2”-(1-hydroxy-1-methylethyl)-dihydrofurano-2,3-h-flavanone; erythro-1-(4-hydroxy-3-methoxyphenyl)-2-{4-(E)-3-hydroxy-1-propenyl-2-methoxy-phenoxy}-1,3-propanediol; 3-2-(4-hydroxyphenyl)-3-hydroxymethyl-2,3-dihydro-1-benzofuran-5-ylpropan-1-ol; 5,7,3’,4’-tetrahydroxy-3-methoxy-8-geranylflavone; 5,7,3’,4’-tetrahydroxy-3-methoxy-8,5’-diprenylflavone; chelerythrine; isoterihanine; β-sitosterol; fucosterol; ergosterol peroxide; D-galacitol; sulfuretin; and graveolone.19
PHARMACOTHERAPEUTIC EFFECTS
Anti-inflammatory activity
Bioactivity-guided fractionation and metabolite study from the methanolic extracts of root bark of Broussonetia papyrifera (L.) L. Her. ex Vent. led to the isolation of twenty compounds; six 1,3 diphenylpropanes, flavanone, two chalcones, five flavans, dihydroflavonol, and five flavonols, including five new compounds. From the screening for inhibition of nitric oxide (NO) and pro-inflammatory cytokines (TNF-α and IL-6) in LPS-stimulated RAW264.7 cells, few compounds exhibited potent anti-inflammatory effects by reducing NO production through downregulating iNOS, COX-2, TNF-α expression, and iNOS protein expression. This study, therefore, reveals that B. papyrifera is a valuable source of phytoconstituents for pharmaceuticals and functional foods for anti-inflammatory diseases such as asthma, chronic obstructive pulmonary disease (COPD), and atopy.20
Anti-SARS CoV-2 activity
A group of polyphenolic compounds was isolated from this medicinal plant of which a chalconoid derivative showed the best inhibitory potential against both Mpro and PLpro (IC50 of 27.9 μM and 112.9 μM, respectively).21
The inhibitory potential of ten polyphenols derived from B. papyrifera roots, i.e. broussochalcone A, broussochalcone B, 4-hydroxyisolonchocarpin, papyriflavonol A, 3'-(3-methylbut-2-enyl)-3’,4,7-trihydroxyflavane, kazinol A, kazinol B, broussoflavan A, kazinol F, and kazinol J were tested against the two SARS CoV proteases with a more potent inhibition recorded against PLpro than that of 3CLpro. The most potent PLpro inhibition was exhibited by the prenylated flavone derivative viz. papyriflavonol A with an IC50 value of 3.7 μM, exceeding the inhibitory potential of non-prenylated flavone derivatives viz. quercetin and kaempferol (IC50 of 8.6 μM and 16.3 μM, respectively). This signified the crucial role of the prenyl group in forming stronger hydrophobic interactions with the enzyme as well as the increase in the hydroxylation in the flavone backbone.22,23
Anti-cancer Activity
The active compounds from B. papyrifera were found to be used for the treatment of human bladder cancer including drug-resistant forms and to establish a potential rationale for their clinical application. The cytotoxic effects of the compound were tested by analyzing cell proliferation, apoptosis, and autophagy where the results suggest that phytoconstituents induces cytotoxic effects in human bladder cancer cells, including the cisplatin-resistant T24R2. The compound may be a candidate for the development of effective anti-cancer drugs on human urinary bladder cancer.24
Anti-nociceptive activity
Various parts of B. papyrifera were studied for its anti-nociceptive and anti-inflammatory activity by chemical-induced pain and inflammation in the rodent model.25 All the parts of B.papyrifera viz., radix, leaf, and fruits effectively inhibit both writhing response induced by 1% acetic acid and late phase licking response caused by 1% formalin. It was observed that radix and fruits reduce the edema induced by 1% carrageenan at 1-2 hrs, also radix reduced the abdominal Evan’s blue extravasations caused by inflammatory mediators including serotonin and sodium nitroprusside. This effect has been attributed due to the presence of one active ingredient, betulinic acid, which inhibited the paw edema caused by serotonin and carrageenan.26
Anti-oxidant activity
Broussochalcone A (BCA), a prenylated chalcone was originally isolated from the cortex of B. papyrifera Vent and the cortex of this plant has been used as traditional medicine for decades.27 BCA is a powerful natural antioxidant that may be primarily attributed due to its free radical-scavenging activity. Moreover, BCA was also found to suppress LPS-induced iNOS protein expression by preventing IkBα degradation in RAW 264.7 macrophages. The free radical-scavenging activity of BCA and its inhibition of iNOS protein expression may have therapeutic potential because excessive free radicals and NO production have been associated with various inflammatory diseases.28
Anti-bacterial Activity
Sohn et al. reported that a prenylated flavonol compound, Papyriflavonol A (Pap A) was isolated from the mulberry roots and evaluated its antimicrobial activity. The results revealed that the minimum inhibitory concentration (MIC) of Pap A against Candida albicans and Saccharomyces cerevisiae were between 10 μg/mL and 25 μg/mL, and its anti-fungal activity was mediated by its ability to disrupt cell membrane integrity. In addition, Pap A had a lower toxic effect than amphotericin B. For the tested strains, the hemolysis ratio of human erythrocytes was less than 5%.29 Geng et al. reported that flavonols in B. papyrifera showed significant in vitro anti-oral microbial activity.30
Anti-proliferative Activity
Guo et al. isolated and purified few active compounds (papyriflavonol A, broussochalcone A, uralenol, broussoflavonol B, and 5,7,3',4'-tetrahydroxy-3-methoxy-8,5'–diprenylflavone) from EtOAc extract of mulberry bark where all of them showed significant anti-proliferative effects on ER-positive breast cancer MCF-7 cells in vitro. The phytocompounds; broussoflavonol B with IC50 = 4.19 μM and 5,7,3',4'-tetrahydroxy-3-methoxy-8,5'-diprenylflavone with IC50 = 4.41 μM were the most effective components than the positive control, icaritin. In an established human breast cancer BCAP-37 xenograft BALB/c nude mice model, broussochalcone A and broussoflavonol B were found to significantly reduce the tumor growth significantly at a concentration of 1 μM by reducing ERK phosphorylation. Western blot indicated that the compounds strongly downregulated the expression of estrogen receptor-α (ER-α).31
Anti-diabetic Activity
Ryu et al. isolated 12 polyphenols from the chloroform extract of the roots of B. papyrifera. Among them, papyriflavonol A (IC50 = 2.1 μM), deoxynojirimycin (IC50 = 3.5 μM), brossoflurenone A (IC50 = 27.6 μM), and brossoflurenone B (IC50 = 33.3 μM) have been identified as potential α-glucosidase inhibitors in comparison to the standard voglibose (IC50 = 23.4 μM). The activity was similar to sugar-derived α-glucosidase inhibitors.32
Lou et al. reported broupapyrin A, a new isoprenylated flavonol isolated from the branches of B. papyrifera in exhibiting a significant inhibitory effect on the well-known anti-diabetic target enzyme PTP-1B with an IC50 value of 0.83±0.30 μM.33
Anti-gout Activity
Researchers found that broussochalcone A (IC50 = 5.8 μM) and 3,4-dihydroxyisolonchocarpin (IC50 = 7.7 μM) were the major contributors to the inhibition of xanthine oxidase. The compound broussochalcone A was identified as the most effective candidate.34
Cytotoxic activity
Ran et al. reported the cytotoxic potentials (against HepG2 cell line) of the compounds (liriodendrin, (+)-pinoresinol-4'-O-β-D-glucopyranosyl-4″-O-β-D-apiofuranoside, and apigenin-6-C-β-D-glycopyranside) that were isolated from the leaves with the IC50 values of 14.56 μg/mL, 19.53 μg/mL, and 17.19 μg/mL, respectively.35
Zhang et al. isolated and reported a new compound altertoxin-IV together with nine known compounds from the ethyl acetate extract (through bioassay-guided fractionation) of a culture of the endophytic fungus Alternaria species G7 present in B. papyrifera. The compounds presented impressive cytotoxic activities against three cancer cell lines (MG-63, A549, and SMMC-7721), of which 3,4',5'-trihydroxy-5-methoxy-6H-benzo[c]chromen-6-one demonstrated noteworthy cytotoxic activity with IC50 values of 2.11 μg/mL, 1.47 μg/mL, and 7.34 μg/mL, respectively. The compound altersolanol A also presented a considerable cytotoxic activity against two cell lines; SMMC-7721 (IC50 = 2.92 μg/mL) and MG-63 (IC50 = 0.53 μg/mL).36
CONCLUSION
This current fascinating article expansively emphasized the general aspects, plant profile (Kingdom, Sub-Kingdom, Infra-Kingdom, Division, Sub-Division, Super-Division Class, Order, Super-Order, Family, Genus, and Species), traditional uses, distribution, major phytoconstituents, significant pharmacotherapeutic attributes (anti-viral, anti-cancer, anti-oxidant, cytotoxic potentials, anti-inflammatory, anti-diabetic, anti-microbial, anti-nociceptive, anti-gout, and anti-proliferative) mediated by diverse parts (seed, root, leaf, stem, and fruit). This information will be reasonably functional for the passionate contemporary investigators of several areas (natural products, pharmacognosy, medicine, chemistry, botany, pharmacy, etc.) in developing miscellaneous essential formulations for treating numerous disorders. This study will pave a new way for modern nature-pharmacotherapeutics for human applications.
ACKNOWLEDGEMENT
None declared.
CONFLICT OF INTEREST
The authors declare no Conflict of Interest regarding the publication of the article.
FUNDING INFORMATION
No funding agency is acknowledged.
AUTHORS CONTRIBUTION
Madhukar Vitthalrao Shende: Physically wrote the full manuscript.
Debarshi Kar Mahapatra: Performed the literature review, set references uniformly, drawn graphical abstract, and prepared the structured abstract.
Atul Arjun Baravkar: Proofread, did necessary changes/corrections, and provided suggestions.
Nilesh Ashokrao Nalawade: Removed plagiarized contents, corrected grammar, and attended all the revisions.
References:
1. Parker RN. A forest flora for Punjab with Hazara and Delhi. Lahore: Government Printing Press; 1956.
2. Watt G. Dictionary of the economic products of India - Volume I. Dehradun: Periodical Experts; 1972.
3. Yu DI, Jing QI, Xiao-yu LI. Research Progress on New Chemical Constituents and Biological Activities of Broussonetia papyrifera. Nat Prod Res Devel. 2014;26(8):1327-31.
4. Qureshi H, Arshad M, Bibi Y. Toxicity assessment and phytochemical analysis of Broussonetia papyrifera and Lantana camara: Two notorious invasive plant species. J Biodivers Environ Sci. 2014;5(2):508-17.
5. Lee D, Bhat KP, Fong HH, Farnsworth NR, Pezzuto JM, Kinghorn AD. Aromatase Inhibitors from Broussonetia papyrifera. J Nat Prod. 2001;64(10):1286-93.
6. Feng W, Li H, Zheng, X. Researches of constituents of Broussonetia papyrifera. Chin J New Drugs. 2008;17:272–8.
7. Sun J, Liu SF, Zhang CS, Yu LN, Bi J, Zhu F, et al. Chemical composition and antioxidant activities of Broussonetia papyrifera fruits. PloS One. 2012;7(2):e32021.
8. Zhang PC, Wang S, Wu Y, Chen RY, Yu DQ. Five New Diprenylated Flavonols from the Leaves of Broussonetia kazinoki. J Nat Prod. 2001;64(9):1206-9.
9. Ji JH, Li H, Kwon Kh SY, Ki HP. Anti-inflammatory activity of the total flavonoid fraction from Broussonetia papyrifera in combination with Lonicera japonica. Korean Soc Appl Pharmacol. 2010;18(2):197-204.
10. Ko HJ, Oh SK, Jin JH, Son KH, Kim HP. Inhibition of experimental systemic inflammation (septic inflammation) and chronic bronchitis by new phytoformula BL containing Broussonetia papyrifera and Lonicera japonica. Biomol Ther. 2013;21(1):66-71.
11. Han Q, Wu Z, Huang B, Sun L, Ding C, Yuan S, et al. Extraction, antioxidant and antibacterial activities of Broussonetia papyrifera fruits polysaccharides. Int J Biol Macromol. 2016;92:116-24.
12. Guo M, Wang M, Deng H, Zhang X, Wang ZY. A novel anticancer agent Broussoflavonol B downregulates estrogen receptor (ER)-α36 expression and inhibits growth of ER-negative breast cancer MDA-MB-231 cells. Eur J Pharmacol. 2013;714(1-3):56-64.
13. Tsai FH, Lien JC, Lin LW, Chen HY, Ching H, Wu CR. Protective effect of Broussonetia papyrifera against hydrogen peroxide-induced oxidative stress in SH-SY5Y cells. Biosci Biotechnol Biochem. 2009;73(9):1933-9.
14. Sohn HY, Son KH, Kwon CS, Kwon GS, Kang SS. Antimicrobial and cytotoxic activity of 18 prenylated flavonoids isolated from medicinal plants: Morus alba L., Morus mongolica Schneider, Broussnetia papyrifera (L.) Vent, Sophora flavescens Ait and Echinosophora koreensis Nakai. Phytomed. 2004;11(7-8):666-72.
15. Chen RM, Hu LH, An TY, Li J, Shen Q. Natural PTP1B inhibitors from Broussonetia papyrifera. Bioorg Med Chem Lett. 2002;12(23):3387-90.
16. Lee D, Bhat KP, Fong HH, Farnsworth NR, Pezzuto JM, Kinghorn AD. Aromatase Inhibitors from Broussonetia p apyrifera. J Nat Prod. 2001;64(10):1286-93.
17. Ryu HW, Curtis-Long MJ, Jung S, Jeong IY, Kim DS, Kang KY, et al. Anticholinesterase potential of flavonols from paper mulberry (Broussonetia papyrifera) and their kinetic studies. Food Chem. 2012;132(3):1244-50.
18. Wang GW, Huang BK, Qin LP. The genus Broussonetia: a review of its phytochemistry and pharmacology. Phytother Res. 2012;26(1):1-10.
19. Qureshi H, Anwar T, Khan S, Fatimah H, Waseem M. Phytochemical constituents of Broussonetia papyrifera (L.) L?He?r. ex Vent: An overview. J. Indian Chem. Soc. 2020;97:55-66.
20. Ryu HW, Park MH, Kwon OK, Kim DY, Hwang JY, Jo YH, et al. Anti-inflammatory flavonoids from root bark of Broussonetia papyrifera in LPS-stimulated RAW 264. 7 cells. Bioorg Chem. 2019;92:103233.
21. Zhou L, Liu Y, Zhang W, Wei P, Huang C, Pei J, et al. Isatin compounds as noncovalent SARS coronavirus 3C-like protease inhibitors. J Med Chem. 2006;49(12):3440-3.
22. Nakao Y, Fujita M, Warabi K, Matsunaga S, Fusetani N. Miraziridine A. A novel cysteine protease inhibitor from the marine sponge Theonella aff. mirabilis. J Am Chem Soc. 2000;122:10462–3.
23. Park JY, Yuk HJ, Ryu HW, Lim SH, Kim KS, Park KH, et al. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J Enzyme Inhib Med Chem. 2017;32(1):504-12.
24. Park S, Fudhaili A, Oh SS, Lee KW, Madhi H, Kim DH, et al. Cytotoxic effects of kazinol A derived from Broussonetia papyrifera on human bladder cancer cells, T24 and T24R2. Phytomed. 2016;23(12):1462-8.
25. Lin LW, Chen HY, Wu CR, Liao PM, Lin YT, Hsieh MT, et al. Comparison with various parts of Broussonetia papyrifera as to the antinociceptive and anti-inflammatory activities in rodents. Biosci Biotechnol Biochem. 2008;72:80276-1-8.
26. Alakurtti S, Mäkelä T, Koskimies S, Yli-Kauhaluoma J. Pharmacological properties of the ubiquitous natural product betulin. Eur J Pharm Sci. 2006;29(1):1-3.
27. Matsumoto J, Fujimoto T, Takino C, Sainto M, Yoshio H, Fukai T, et al. Components of Broussonetia papyrifera (L.) VENT. I. Structures of two new isoprenylated flavonols and two chalcone derivatives. Chem Pharm Bull. 1985;33:3250–6.
28. Cheng ZJ, Lin CN, Hwang TL, Teng CM. Broussochalcone A, a potent antioxidant and effective suppressor of inducible nitric oxide synthase in lipopolysaccharide-activated macrophages. Biochem Pharmacol. 2001;61(8):939-46.
29. Sohn HY, Kwon CS, Son KH. Fungicidal effect of prenylated flavonol, papyriflavonol a, isolated from broussonetia papyrifera (L.) vent. against Candida albicans. J Microbiol Biotechnol. 2010;20(10):1397-402.
30. Geng CA, Yan MH, Zhang XM, Chen JJ. Anti-oral microbial flavanes from Broussonetia papyrifera under the guidance of bioassay. Nat Prod Bioprospect. 2019;9(2):139-44.
31. Guo F, Feng L, Huang C, Ding H, Zhang X, Wang Z, et al. Prenylflavone derivatives from Broussonetia papyrifera, inhibit the growth of breast cancer cells in vitro and in vivo. Phytochem Lett. 2013;6(3):331-6.
32. Ryu HW, Lee BW, Curtis-Long MJ, Jung S, Ryu YB, Lee WS, et al. Polyphenols from Broussonetia papyrifera displaying potent α-glucosidase inhibition. J Agric Food Chem. 2010;58(1):202-8.
33. Lou Y, Su SY, Li YN, Lei C, Li JY, Hou AJ. Flavonoids with PTP1B inhibition from Broussonetia papyrifera. Chin J Chin Mater Med. 2019;44(1):88-92.
34. Ryu HW, Lee JH, Kang JE, Jin YM, Park KH. Inhibition of xanthine oxidase by phenolic phytochemicals from Broussonetia papyrifera. J Korean Soc Appl Biol Chem. 2012;55(5):587-94.
35. Xiao-Ku R, Xiao-Tong W, Pei-Pei L, Yu-Xin C, Bo-Jia W, De-Qiang D, et al. Cytotoxic constituents from the leaves of Broussonetia papyrifera. Chin J Nat Med. 2013;11(3):269-73.
36. Zhang N, Zhang C, Xiao X, Zhang Q, Huang B. New cytotoxic compounds of endophytic fungus Alternaria sp. isolated from Broussonetia papyrifera (L.) Vent. Fitoterapia. 2016;110:173-80.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License