IJCRR - 13(12), June, 2021
Pages: 09-15
Date of Publication: 22-Jun-2021
Print Article
Download XML Download PDF
Micro-RNA 155 - On the Crosspoint of Inflammation and Carcinogenesis
Author: Antonia Atanassova, Avgustina Georgieva, Trifon Chervenkov, Assia Konsoulova
Category: Healthcare
Abstract:Background: As miR-155 influences a wide spectrum of inflammatory mediators, the study of this miRNA may suggest a new insight over the cancer development mechanisms. This is why the investigation of the miR-155 expression may reveal a potential relation between inflammation and gastrointestinal cancer. The aim of the current study is to assess the miR-155 expression levels in patients with Crohn's disease (CD) and ulcerative colitis (UC). Materials and Methods: The expression of miR-155 was studied in 70 consecutive patients with a confirmed diagnosis of IBD: 35 with CD and 35 with UC and 30 healthy controls. Disease activity was evaluated by the clinical symptoms, biochemical inflammatory parameters (CRP, FCP) and validated indices for evaluating IBD activity (CDAI for CD, Montreal Classification, and partial Mayo score for UC). 25(OH)D serum concentrations were measured by a commercial paramagnetic particle chemiluminescent immunoassay for the quantitative determination of total 25 - hydroxyvitamin D [25(OH) vitamin D] levels use on Access 2 Immunoassay Systems. Serum expression of miR-155 by reverse transcriptase, a real-time quantitative polymerase chain reaction (RT-qPCR), was tested in all. Results: The analysis of the results showed that the circulating miR-155 was increased in Crohn-colitis (3.51\?5.22) and extensive UC (2.86\?5.44). Levels of CDAI above 150 were a risk factor for detection of increased miR-155 expression levels (OR=10, 91 (1.194-99,688); p=0.017). An increased miR-155 expression was detected in patients, treated with corticosteroids (5.20\?8.91 for UC and 3.39\?3.10 for CD). There was an inverse proportional moderate correlation with the levels of FCP (r= -0.344 :p< 0.05). Vitamin D deficiency (25(OH)D) led to a 1.24 higher risk for increased serum level of miR-155. Conclusions: The increased expression of miR-155 in patients with IBD is seen in disease localization in the colon, persistent inflammation, severe disease activity, vitamin D deficiency and treatment with corticosteroids.
Keywords: Fecal calprotectin, CRP, Crohn’s disease, micro-RNA 155, Ulcerative colitis, Vitamin D
Full Text:
Introduction:
Micro RNAs (miRNAs) are endogenous non-coding RNAs (ncRNAs), with a length of about 22 nucleotides. The biogenesis of the miRNAs develops in several steps of post-transcriptional modifications in the nucleus and the cytoplasm.1 Over 60% of all genes, that code proteins, are regulated by miRNAs 2,3,4 and one miRNA has on average 200 targets. [5,6,7]
miRNAs are important regulators of different cellular processes, including development, differentiation and signalization.8-12 The dysregulation of specific miRNAs may lead to different diseases in humans: metabolic disturbances, cardiovascular or liver diseases as well as immune dysfunction, including the development of neoplasms.13-18
The expression of miRNA-155 (miR-155) is related to different cardiovascular diseases, inflammation and cancers. Its multifunctional regulatory role defines the interest towards this particular miRNA, which is considered to be the most extensively studied.
Expression of miR-155 was first reported in the human spleen and thymus and subsequently in the liver, lungs and kidneys [19, 10 Later on, it was established that this miRNA has an abnormally increased expression in different activated immune cells [21], which defines the important role of miR-155 in immune response.22,23,24
miR-155 responds to many inflammatory stumuli as tumor necrosis factor alfa (TNF- α), interleukin (IL) -1b, interferons, pathogen-associated molecular patterns (PAMP), damage- associated molecular patterns, (DAMP) [25] , alarmins (e.g. IL-1a) [26] , hypoxia [27] , as well as in Toll-like receptor ligans (TLR) in monocytes and macrophages [28] B cells.29
The expression of miR-155 is controlled by multiple signal pathways. The regulatory cytokines, including Transforming Growth Factor beta (TGF-β) may induce or inhibit the expression of miR-155 30,31,32 IL-10 decreases its expression via inhibition of the transcription factor Ets2.
In IBD, the negative feedback control over the inflammation is disturbed, leading to excessive activation of the inflammatory signal pathways. The suppressor of the cytokine signalization 1 (SOCS1) is a critical negative regulator, blocking the Janus kinases (JAKs), signal transducer and activator of transcription proteins (STATs)- JAK / STAT pathway [33] and suppressing the interleukin-1 receptor-associated kinase 4 (IRAK4) in the Toll-like receptor 4 (TLR4) signal pathway. 34 The rapid increase of miR-155 suppresses the translation of SOCS1 during the endotoxin-induced inflammatory reaction and thus avoiding any suppression of the ongoing inflammatory cascade. 34,35
The endogeneous and the synthetic glucocorticoids are highly effective to slow down the process of acute inflammation via suppression of the miR-155 expression in the glucocorticoid receptor and/or via nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB). 36,37 The expression of MiR-155 is regulated by the associated with the immune response transcription factor forkhead box P3 (FOXP3), which regulates the functioning of the T regulatory (Treg) cells. Moreover, miR-155 regulates the special AT-rich sequence-binding protein-1 (SATB1) and Zinc finger E-box-binding homeobox 2 (ZEB2) expression levels in the Treg cells. 38
Chen Y, et.al, 2013 (39) identified that the vitamin D receptor signal pathway blocks the activation of NF-kB and thus leads to a decrease in miR-155 levels. As a result of this, the translation of SOCS1 is increased, allowing for an increase in the regulation of the negative feedback over the immune response. 40
At least 15-20% of all types of human cancer are related to chronic inflammation: diseases as IBD, colorectal cancer, colitis-associated cancer (in ulcerative colitis), chronic gastritis and H. pylori (gastric cancer, GC).41 As miR-155 influences a wide spectrum of inflammatory mediators, the study of this micro-RNA may suggest a new insight into the cancer development mechanisms. This is why the investigation of the miR-155 expression may reveal a potential relationship between inflammation and gastrointestinal cancer.
The current study aims to assess the miR-155 expression levels in patients with Crohn’s disease (CD) and ulcerative colitis (UC).
Materials and methods: The expression of miR-155 was studied in 70 consecutive patients with a confirmed diagnosis of IBD: 35 with CD and 35 with UC. Patients were treated at the Gastroenterology clinic for a period of one year (April 2019 – April 2020). 30 healthy individuals were also tested to define a healthy control study group. All IBD patients were classified according to the Montreal classification. The clinical course and treatment regimens were assessed. Disease activity was evaluated by the clinical symptoms, biochemical inflammatory parameters (C reactive protein - CRP, faecal calprotectin - FCP) and validated indices for evaluating IBD activity (CDAI for CD, Montreal Classification, and partial Mayo score for UC). Biochemical parameters (CRP and FCP) were assessed as either normal or abnormal: FCP was considered as normal if levels were < 50 mg/g; CRP was considered abnormal (elevated) if measured > 5 mg/l. 25(OH)D serum concentrations were measured by a commercial paramagnetic particle chemiluminescent immunoassay for the quantitative determination of total 25-hydroxyvitamin D [25(OH) vitamin D] levels use on Access 2 Immunoassay Systems. Vitamin D deficiency was defined as a serum level of 25OHD lower than 50 nmol/L; serum level above 50 nmol/L but lower than 75 nmol/L were classified as vitamin D insufficiency.
Levels of miR-155 were assessed in blood serum. 5 ml of blood was obtained via peripheral venous puncture with closed system BD Vacutainer™ SST™ II Advance (Becton Dickinson, USA). After withdrawal, the blood sample was held 30 minutes at room temperature for clothing. Subsequently, it was centrifuged at 500×g for 15 minutes at room temperature and the serum was separated and divided into aliquots of 500 µl that were immediately stored at −80 °C until the moment of the analysis.
miRNAs were isolated from 200 µl serum using a pre-existing commercial miRNeasy Serum/Plasma Kit (50), catalogue ?217184 (QIAGEN, Germany) as per the protocol of the manufacturer. 3,5 μl (1,6×108 copies per µl) control miRNA C. elegans miR-39: miRNeasy Serum/Plasma Spike-In Control, catalogue ?219610 (QIAGEN, Germany), was added to each sample for normalization control; the samples were afterwards eluted in 14 µl RNA-ase free water.
Each of the samples was subsequently submitted to reverse transcription via ready-to-use commercial kit miScript II RT Kit (50), catalogue ?218161 (QIAGEN, Germany) as per manufacturer’s protocol from 2,5 µl eluted miRNA in a final volume of 10 µl with HiFlex buffer and it was incubated at 37 °C for 60 minutes and the enzyme was inactivated at 95 °C for 5 minutes.
Each of the samples was then submitted to a quantitative real-time polymerase chain reaction (rt-PCR) via a ready-to-use commercial kit miScript SYBR Green PCR Kit (200), catalogue ? 218073 (QIAGEN, Germany) and prepared primers miScript Primer Assay (100), catalogue ?218300 (QIAGEN, Germany) as per manufacturer’s protocol: 1 µl complementary DNA (cDNA) in 10 µl reactions in 3-times repetitions for 15 target miRNA in 384 well plates. The used miScript Primer Assay primers (100), catalogue ? 218300 (QIAGEN, Germany) were as follows (the reference number is in the brackets): Ce_miR-39_1 (MS00019789), Hs_miR-155_2 (MS00031486), Hs_RNU6-2_11 (MS00033740). The used temperature parameters were as follows: maintenance for 15 minutes at 95 °C for enzyme activation; 40 cycles of 15 seconds at 94 °C; 30 seconds at 70 °C with fluorescent reading; analysis of the melting curve to prove the specificity of the amplification: primary denaturation for 15 seconds at 95 °C and cooling to 55 °C for 60 seconds with an increase to 95 °C with velocity of +0,05 °C per second and fluorescent reading. The analysis was done by QuantStudio Dx instrument of Applied Biosystems (USA) company; a threshold cycle (Ct) was assessed for each sample. Receiver Operating Characteristic Curve (ROC) was calculated to detect diagnostic performance of the test, sensitivity, specificity, positive and negative predictive values. The significance of the obtained results was judged at the 5% level.
The results were calculated with SPSS, v. 20.0 for Windows. We used variation, correlation, regression analyses as well risk assessment and comparative analyses (c2, t-test, ANOVA). ?<0.05 was used as a level of significance.
The clinical trial was initiated after approval and permission ?82 / 28.03.2019 of the Ethics Commission for scientific research at the Medical University – Varna, Bulgaria.
Results: Table 1 shows the characteristics of the patients with CD, UC and the healthy control subjects.
As there are no validated referent thresholds for the expression of any micro RNA, we calculated a cut-off threshold level to differentiate between healthy control subjects and patients with chronic inflammatory bowel diseases (CIBD). This cut-off is specific for our cohort of studied healthy subjects and was used as a marker for assessment of the miR-155 expression in patients with UC and CD. In our study, the cut-off value for the miR-155 is calculated at 1.37 (AUC 0.620; 0.497-0.742) with a sensitivity of 61.4 % and specificity of 63.3% (Fig. 1).
Table 2 shows the expression of miR-155 according to the characteristics of the patients with CD and UC.
The analysis of the results of the miR-155 according to the localization of the disease indicates that the circulating miR-155 is increased in Crohn-colitis (3.51±5.22) and extensive UC (2.86±5.44). In patients with CD, there is no significant difference in the miR-155 expression levels in inflammatory (?1) or stenotic (?2) form of the disease, whereas, in patients with UC, not achieving a significant remission (chronic persistent form), the expression of miR- 155 is significantly higher as compared to patients with a chronic relapsing course of disease (5.53±9.56 vs 1.54±1.22; P=0.032).
The correlation analysis shows a proportional moderate correlation between miR-155 expression CDAI (r=0.415; p=0.015). This indicates that the progression of the disease activity leads to an increase in the serum expression levels of miR-155. Patients with moderate CD activity have significantly increased expression of miR-155 as compared to patients in remission or with mild activity (6.08±5.12 vs 2.11±2.09 and 2.11±1.07; ?=0.009). Levels of CDAI above 150 are a risk factor for detection of increased miR-155 expression levels (OR=10,91 (1.194-99,688); p=0.017).
The analysis of the correlation between the severity of the UC and the expression of micro-RNA 155 shows that the severity index S (as per the Montreal classification) has a proportional moderate correlation with the increased expression of miR-155 (r=0.321; p=0.048). Patients with severe disease activity have a significantly higher expression level of miR-155 (?<0.05). On the other hand, the endoscopic activity, measured by the ?artial Mayo score, does not show a correlation with the miR-155 expression – the miR-155 levels are increased in patients with moderate disease activity (4.82±9.05) and decreased in patients with severe activity (2.23±1.75). In patients with UC in remission or with mild disease activity, the miR-155 expression levels are close or just mildly below the threshold cut-off value, defined for the healthy control subjects.
Regarding the treatment, an increased miR-155 expression is detected in patients, treated with corticosteroids (5.20±8.91 for UC and 3.39±3.10 for CD). The expression of miR- 155 is similar in patients, treated with immune modulators in both CD and UC. In patients with UC, receiving biological treatment, the levels of serum miR-155 expression are below the defined cut-off value for healthy controls.
Other widely used biomarkers, used to biochemically assess inflammation in patients with IBD, are CRP and FCP. The mean level of CRP levels in patients with IBD is 19.31 mg/l
± 32.69 mg/l (0.09-160.0 mg/l), and for FCP it is 459.19 mg/g ± 490.74 mg/g (2.00 – 1 800 mg/g). The results of the analysis of the correlation between the miR-155 expression and CRP or FCP shows that the levels of the tested micro-RNA do not correlate with the levels of CRP, but there is an inverse proportional moderate correlation with the levels of FCP (r=-0.344 :p<0.05).
The mean 25(OH)D serum expression level in patients with IBD is 44.14 nmol/L ±17.47 nmol/L with a variation between 2.76 nmol/L and 90.65 nmol/L. These results show that the expression of miR-155 is different according to the serum levels of 25(OH)D. In patients with 25(OH)D serum levels < 50 nmol/L, the expression of miR-155 is significantly higher as compared to patients with 25(OH)D levels ≥ 50 nmol/L (3.53±6.35 vs 2.38±2.23; ?=0.041). Vitamin D deficiency (25(OH)D) leads to a 1.24 higher risk for increased serum level of miR- 155.
Discussion: Many studies show that the miR-155 expression is increased in patients with IBD, both in UC and CD.42,43,44 Our results also correspond with previous publications, showing a significantly higher miR-155 expression level in patients with IBD as compared to healthy individuals. In the study of Béres et al. 45 in the pediatric population with UC and CD, the authors suggest that the miR-155 expression is significantly higher in areas of mucosal inflammation in comparison with healthy controls.[46] Similar results, showing increased miR-155 levels in patients with gastric and duodenal H. Pylori infection are reported by Wan J et al.47
The expression of miR-155 in patients with active UC is increased in comparison to healthy individuals. This is a result of the direct decrease in the expression of the FOXO3a gene which may additionally activate the NF-κB signalling pathway and increase the inflammatory cytokines and thus – maintain inflammation.48,49 These results are confirmed by our study as well.
It is interesting that in gastric cancer cell lines and tissue models, the expression of miR- 155 decreases. The tumorigenesis is a complex process and may undergo via silencing or loss of the miR-155 expression.47 Meanwhile, in patients with CRC there is an overexpression of miR-155.50,51
In our trial, patients with persistent inflammation and no remission as well as localization of their disease in the colon, have a significantly higher miR-155 expression. It is well known that chronic inflammation is the background of the IBD, UC-associated carcinoma as well of colorectal cancer.41 Logically, the change in the expression of miR-155 levels during monitoring of inflammatory processes in IBD could be a potential biomarker for the early discovery of dysplasia. Further larger prospective cohort trials in IBD patients are needed to explore the role of miR-155 in tumorigenesis and to validate the change in its expression levels as a potential biomarker for the occurrence of dysplasia and its transition into tumorigenesis. There are publications that the localization and the disease course may be related to different miR-155 levels. Increased miR-155 levels in intestinal myofibroblasts and the mucosa of the colon are related to the control of the Th17 inflammatory pathway, targeting FOXO3a and IL-8, thus promoting fibrosis formation.52,53
It has been proven that miR-155 plays a significant role in the control of inflammation and immune regulation and it is logical that its expression is significantly higher in patients with active disease, regardless of the method, assessing the activity of the disease.
Chen Y. et.al. describes a new mechanism, where the vitamin D receptor signalling pathway controls the TLR4-mediated inflammation in IBD and the immune-mediated diseases. This anti-inflammatory mechanism is mediated via miR-155 suppressing the regulation, decreasing SOCS1. This leads to enhancement of the regulation of the negative feedback. Vitamin D – receptor signalling pathway inhibits miR-155 via a transcription mechanism.[39] This process of suppression of the miR-155 expression is also confirmed in our study where we established an inverse proportional correlation between the vitamin D levels and the miR-155 expression. Vitamin D deficiency correlates with higher miR-155 expression levels.
Treatment with 5-ASA and biologicals is highly effective in patients with UC and leads to a decrease in the miR-155 expression to levels, close to or below the threshold cut-off values as in healthy individuals. On the other hand, in patients with CD, treated with 5-ASA, the levels of miR-155 do not decrease to such extent which shows the persistence of the inflammation and explains the difficult achievement of remission in patients with CD.
Treatment with corticosteroids is highly effective in suppressing acute inflammation via a change in the miR-155 expression. [36,37] In our cohort of patients, treated with corticosteroids to induce remission, the levels of miR-155 are increased and this may be explained by the fact that the disease remission, induced by corticosteroids is short-term and unstable as there is a high risk of recurrence or complications of the disease.
Conclusion: The increased expression of miR-155 in patients with IBD is seen in disease localization in the colon, persistent inflammation, severe disease activity, vitamin D deficiency and treatment with corticosteroids. Decreased miR-155 expression with levels, close to the levels of healthy subjects is reported predominantly in patients with UC in remission, induced by biological therapy. As miR-155 has a significant role in the assessment of the inflammatory process and plays an important role in the tumorigenesis, longer monitoring of the dynamics of its expression in patients with active IBD may reveal a potential crosspoint between inflammation and gastrointestinal cancer.
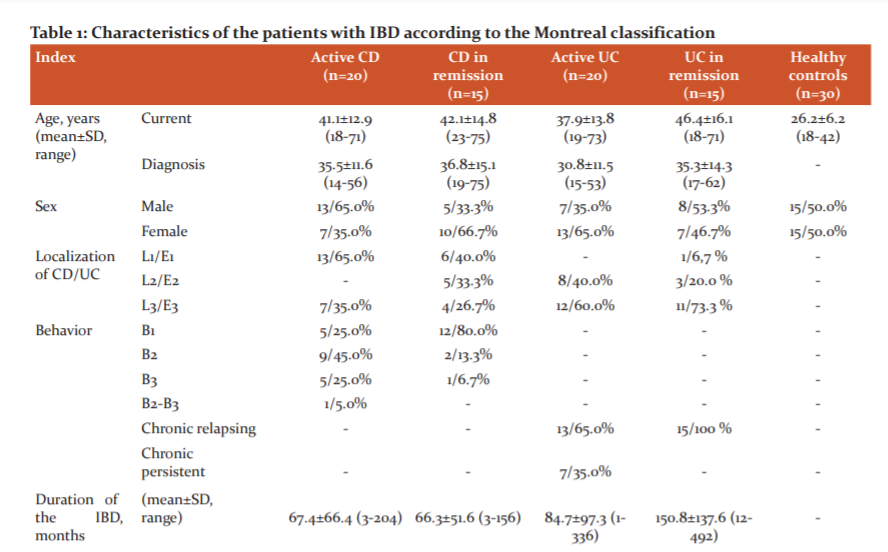

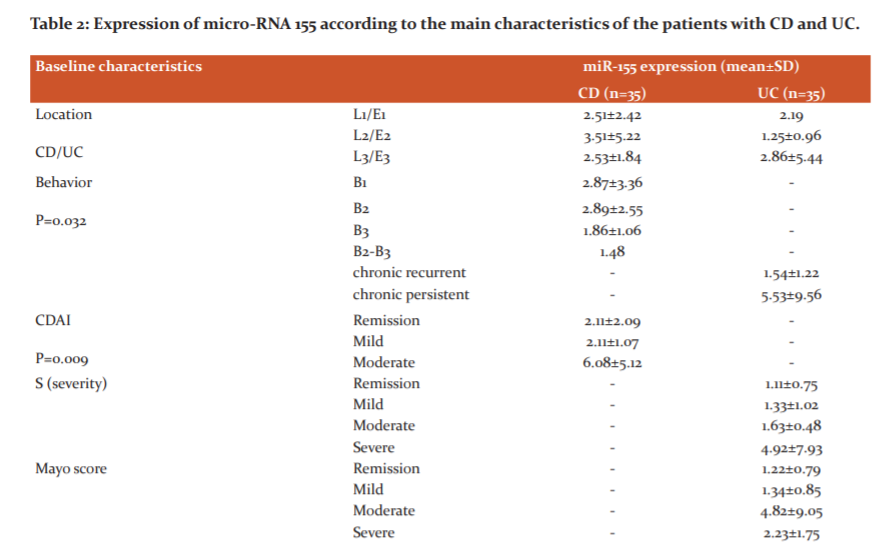
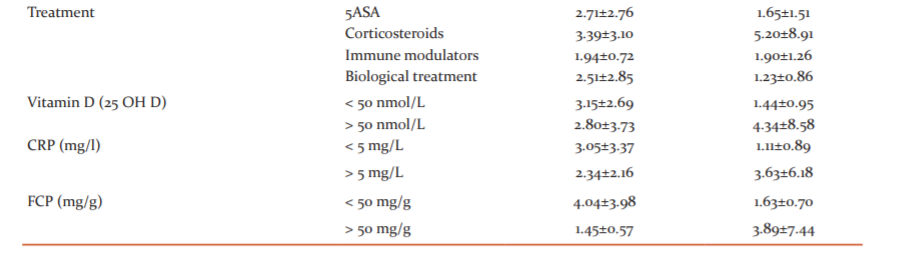
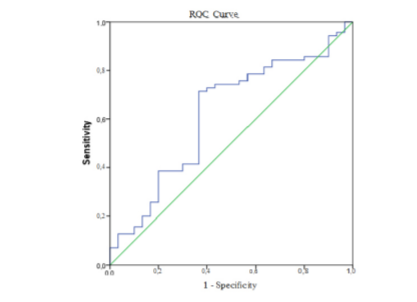
Fig. 1. A receiver operating characteristic curve (ROC curve) for serum miR-155 expression to predict cases (from control).
References:
1. Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004 Nov 11;432(7014):231-235.
2. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004 Jan 23;116(2):281-297.
3.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009 Oct;10(10):704-714.
4. Lin PY, Yu SL, Yang PC. MicroRNA in lung cancer. Br J Cancer. 2010; 103(8):1144–1148.
5. Bagga S, Bracht J, Hunter S, Massirer K, Holtz J, Eachus R,et al. Regulation by let-7 and lin-4 miRNAsresults in target mRNA degradation. Cell. 2005; 122(4):553–563.
6. Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, et al. Combinatorial microRNA target predictions. Nat Genet. 2005; 37(5):495–500.
7. John B, Sander C, Marks DS. Prediction of human microRNA targets. Methods Mol Biol. 2006; 342:101–113.
8. Ambros V. The functions of animal microRNAs. Nature. 2004; 431(7006):350–355.
9. Bagga S, Pasquinelli AE. Identification and analysis of microRNAs. Genet Eng. 2006; 27:1–20.
10. Hwang HW, Mendell JT. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer. 2006; 94(6):776–780.
11. Du T, Zamore PD. Beginning to understand microRNA function. Cell Res. 2007; 17(8):661–663.
12. Rane S, Sayed D, Abdellatif M: MicroRNA with a MacroFunction. Cell Cycle. 2007; 6(15):1850–1855.
14. Poy MN, Spranger M, Stoffel M. microRNAs and the regulation of glucose and lipid metabolism. Diabetes Obes Metab. 2007; 9 Suppl 2:67–73.
17. Blenkiron C, Miska EA. miRNAs in cancer: approaches, aetiology, diagnostics and therapy. Hum Mol Genet. 16 Spec No 2007; 1:R106–R113.
18. Van Rooij E, Olson EN. MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest. 2007; 117(9):2369–2376.
19. Tam W. Identification and characterization of human BIC, a gene on chromosome 21 that encodes a noncoding RNA. Gene. 2001; 274(1–2):157–167.
21. Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007; 129(7):1401–1414.
22. Costinean S, Zanesi N, Pekarsky Y, Tili E, Volinia S, HeeremaN, Croce CM. Pre-B cell proliferation and lymphoblastic leukaemia/high-grade lymphoma in E(mu)-miR155transgenic mice. Proc Natl Acad Sci. U S A 2006; 103(18):7024–7029.
25. O’Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci. 2007; 104(5):1604–1609.
26. Kurowska-Stolarska M, Hasan MK, Welsh DJ, Stewart L, McIntyre D, Morton BE, et al. The role of microRNA-155/liver X receptor pathway in experimental and idiopathic pulmonary fibrosis. J Allergy Clin Immunol. 2017; 139(6):1946–1956.
27. Bruning U, Cerone L, Neufeld Z, Fitzpatrick SF, Cheong A, Scholz CC, et al. MicroRNA-155 promotesresolution of hypoxia-inducible factor 1alpha activity during prolonged hypoxia. Mol Cell Biol. 2011; 31(19):4087–4096.
28. Mashima R. Physiological roles of miR-155. Immunology. 2015; 145(3):323–333.
31. Pottier N, Maurin T, Chevalier B, Puissegur MP, Lebrigand K, Robbe-Sermesant K, et al. Identification of keratinocyte growth factor as a target of microRNA-155 in lung fibroblasts: implication in epithelial-mesenchymal interactions. PLoS One. 2009; 4(8):e6718.
32. Valeri N, Gasparini P, Fabbri M, Braconi C, Veronese A, LovatF, et al. Modulation of mismatch repair and genomic stability by miR-155. Proc Natl Acad Sci. 2010; 107(15):6982–6987.
33. Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nature Revi Immun. 2007; 7, 454-465.
35. Alhassan Mohammed H, Mirshafiey A, Vahedi H, Hemmasi G, Moussavi Nasl Khameneh A, Parastouei K, et al. Immunoregulation of Inflammatory and Inhibitory Cytokines by Vitamin D3 in Patients with Inflammatory Bowel Diseases. Scand J Immunol. 2017 Jun;85(6):386-394.
36. Zheng Y, Xiong S, Jiang P, Liu R, Liu X, Qian J, et al. Glucocorticoids inhibit lipopolysaccharide-mediated inflammatory response by downregulating microRNA-155: a novel anti-inflammation mechanism. Free Radic Biol Med. 2012; 52(8):1307–1317.
37. Chinenov Y, Coppo M, Gupte R, Sacta MA, Rogatsky I. Glucocorticoid receptor coordinates transcription factor-dominated regulatory network in macrophages. BMC Geno-mics. 2014; 15:656.
38. Brown CY, Dayan S, Wong SW, Kaczmarek A, Hope CM, Pederson SM, et al. FOXP3 and miR-155 cooperate to control the invasive potential of human breast cancer cells by downregulating ZEB2 independently of ZEB1. Oncotarget. 2018; 9(45):27708–27727.
39. Chen Y, Liu W, Sun T, Huang Y, Wang Y, Deb DK, et al. 1,25-dihydroxyvitamin D promotes negative feedback regulation of TLR signalling via targeting microRNA-155-SOCS1 in macrophages. J Immunol. 2013 Apr 1;190(7):3687-3695.
40. Hart PH, Gorman S, Finlay-Jones JJ. Modulation of the immune system by UV radiation: more than just the effects of vitamin D? Nat Rev Immun 2011; 11, 584-596.
41. Rath T, Billmeier U, Waldner M.J, Atreya R, Neurath MF. From physiology to disease and targeted therapy: Interleukin-6 in inflammation and inflammation-associated carcinogenesis. Arch. Toxicol. 2015; 89,541–554.
42. Fasseu M, Tréton X, Guichard C, Pedruzzi E, Cazals-Hatem D, Richard C, et al. Identification of restricted subsets of mature microRNA abnormally expressed in the inactive colonic mucosa of patients with inflammatory bowel disease. PLoS One. 2010 Oct 5;5(10):e13160.
43. Takagi T, Naito Y, Mizushima K, Hirata I, Yagi N, Tomatsuri N, et al. Increased expression of microRNA in the inflamed colonic mucosa of patients with active ulcerative colitis.J. Gastroenterol. Hepatol. 2010; 25, S129–S133.
44. Wu F, Zhang S, Dassopoulos T, Harris ML, Bayless TM, Meltzer SJ, et al. Identification of microRNAs associated with ileal and colonic Crohn's disease. Inflamm Bowel Dis. 2010 Oct;16(10):1729-38.
49. Kaser A, Zeissig S. Blumberg, R.S. Inflammatory bowel disease.Annu. Rev. Immunol. 2010; 28, 573–621.
52. Min M, Peng L, Yang Y, Guo M, Wang W, Sun G. MicroRNA-155 is involved in the pathogenesis of ulcerative colitis by targeting FOXO3a. Inflamm Bowel Dis. 2014; 20(4):652–9.
53. Pathak S, Grillo AR, Scarpa M, Brun P, D’Incà R, Nai L, et al. MiR-155 modulates the inflammatory phenotype of intestinal myofibroblasts by targeting SOCS1 in ulcerative colitis. Exp Mol Med. 2015; 47: e164. doi: 10.1038/emm.2015.21.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License