IJCRR - 13(11), June, 2021
Pages: 53-58
Date of Publication: 04-Jun-2021
Print Article
Download XML Download PDF
Zebrafish: A New Emerging Model of Experimental Pharmacology
Author: More SM, Layar A, Darade S, Shahu A, Kushwaha N, Kharwade RS, Mahajan UN
Category: Healthcare
Abstract:Zebrafish as the new emerging model is used to detail the study of the zebrafish, Taxonomy, Developmental stages of zebrafish. The various experimental model is used in the detection of the various function in the body, while In Vivo and In Vitro study, both studies are done under appropriate environment and proper procedure. Our present study mainly developing and testing medicines and vaccines for humans and other animals, studying how animals and human bodies function, Assessing the safety of chemicals such as pesticides for their possible effect on human health or the environment. In Pharmacological screening, various disease and disorder can be studied from the major disease which shows the better result in pharmacological screening. Teratogenicity is the most useful study as the embryo develops outside the body and the study of the embryo is done through the microscope. The other major property of zebrafish is the regeneration of the heart where the cardiovascular disease in the regeneration of the heart is studied. In Type-2 Diabetes Mellitus, the different test is studied to examine the abnormalities produce in the blood of zebrafish and the various effect of the drug which control the blood glucose level in the blood of zebrafish.
Keywords: Zebrafish, Teratogenicity, Animal model, Pharmacological screening
Full Text:
INTRODUCTION
Experimental Pharmacology is the study of the interaction between the leaving organism and the chemicals that affect normal or abnormal biochemical function. Experiment Pharmacology mainly deals with the drug which is either man-made or endogenous molecule which examines the biochemical or physiological effect. The discovery of new drug or detail study of existing is also coming under experimental pharmacology.1,2
In-vivo Study
The word In Vivo comes from Latin which means “Within the leaving”. In the controlled environment experiments and observation is taken on the living tissue of the whole leaving organism. Any drug discover lead to the clinical trial or chemical testing in the appropriate condition is given in proper concentration of drug to the animals like rats, rabbit, fish and other animals after that human trial is performed.3
In-vitro Study
The word In Vitro comes from Latin that means “Within the glass”, which means the study is done outside the living organism The term in vitro in cell biology explain the technique which is performed in the controlled environment outside the body like in a test tube, Petri dishes or any other. The in vitro method is not much expensive as compared to the in vitro and gives quick result.4
Experimental animals
Experimental animals are the animals that we are used for our research purpose to determine the different activities of Drugs (Table 1). Mainly developing and testing medicines and vaccines for humans and other animals, studying how animals and human bodies function, Assessing the safety of chemical such as pesticides for their possible effect on human health or the environment.6

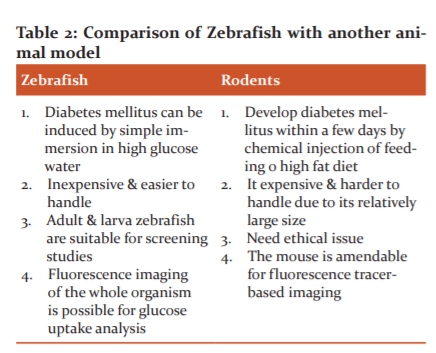
Advantages of Zebrafish as organ model as compared to other model shown in Table 2 and following are the characteristics feature which makes zebrafish as the best model for experimental pharmacology.
-
Small size
-
Rapid embryonic development
-
Short generation time
-
External fertilization and development
-
High fecundity
-
Optical transparency of embryos and larvae
-
Low husbandry cost
-
Fully sequenced genome sequence
-
High genetic homology with human
-
Orthologues genes between human and zebrafish
-
Large-scale mutagenesis
-
Transgenic models available
-
High-throughput genetic/drug screening
-
Direct administration of compounds to the medium of embryos
-
Genetic manipulations/engineering (forward and reverse genetics)
-
Excellent models for developmental toxicology studies.7,8
ZEBRAFISH ANIMAL MODEL CHARACTERISTICS
Danio rerio, commonly it is also known as Zebrafish, belongs to the minnow family. To study fundamental biological questions, it is considered one of the most popular model organisms. It is a small 1 to 1.5 inches fish that can be grown easily in an aquarium. Genetic similarity with a human being (about 70-80%) Breed readily (nearly every 10 days) & can produce 50 to 300 eggs at a time.9,10
Taxonomy
The zebrafish is a derived member of the genus Brachydanio, of the family Cyprinidae. Zebrafish are demonstrated by the phylogenetic tree of close species which is closely related to the genus Devario. Recent molecular studies have suggested that it should belong to the genus Brachydanio as ‘Brachydanio rerio’
Developmental stages of Zebrafish (life cycle)
In Figure 1 complete development stage with life cycle explain. Figure 2 shows the breeding method of zebrafish. Zebrafish lifecycle divided into four major periods. Embrio, larva, juvenile and adult. The full life cycle from fertilised egg to adult is a quick 90 days. Early development occurs at a rapid, but predictable rate when the embryos are raised at 28oC. 11,12

ZEBRAFISH : ANIMAL MODEL IN DIFFERENT DISEASE CONDITION
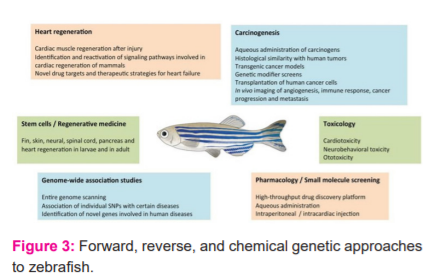
Zebrafish as experimental animal model use in A) The Zebrafish Embryo Toxicity & Teratogenicity, B) Cardiovascular disease, C) Type 2 Diabetes Mellitus shows in Figure 3.
-
The Zebrafish Embryo Toxicity & Teratogenicity
Teratogenicity is the abnormality in the fatal when drug administered to pregnant women, while in zebrafish it has recently emerged for the organism for genetic research & vertebrate development. As the zebrafish have the major advantage in external development of embryo which allows direct observation through Stereo Microscope and also the teratogenic activity is directly seen from the microscope. The embryo Toxicity and Teratogenicity of the drug at what concentration is seen. 13,14
Method
After the breading period of zebrafish, the egg/embryo is collected and strain in a soft mesh sieve after that transfer it into a clean embryo medium. The evaluation of the egg/embryo is done by the embryo which is capable to survive, and the unfertilized egg/embryo is taken off. Incubate the egg at 28.5ºc for 2-3 hr the chorion (soft eggshell) is getting soften and it will get remove, observe under the stereo-microscope and completely remove corn is selected after that the selected embryo is transferred into a single well of multiwall tissue and it is filled with a drug to be tested at various concentration along with vehicle and warm the plate at 28.5ºc for 5 days, the development of the embryo is seen through a microscope at different period, the larva start movement at 3rd -4th day. The endpoint analysis is done period time 2nd -3rd& 4th-day post-fertilization after complete analysis euthanize the larva by immersion of 1.2% sodium hypochlorite (bleach) solution for around 4-7days.13-16
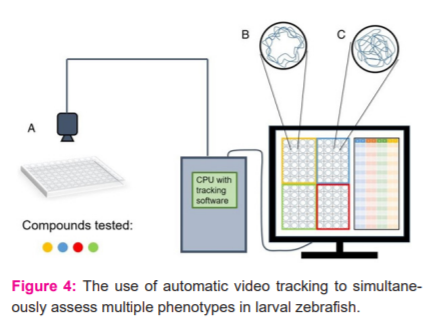
Teratogenicity is the abnormality in the physiological development of the fetus. In teratology, the zebrafish model shows the best model. Figure 4 shows the use of automatic video tracking to simultaneously assess multiple phenotypes in larval zebrafish. Panel (A) shows a 96?well holding plate to administer several compounds to larval zebrafish. Fish behaviours are recorded by an overhead camera, and images are processed through tracking software. Panel (B) showing an example of a swim trace. In this, the larval zebrafish stay close to the walls that are wall?hugging behaviour. Panel (C) showing an example of the swim pattern (opposite). In this, the larval zebrafish prominently explore the environment, involving the centre of the tank. 14,15
-
Cardiovascular Disease (CVD)
Cardiovascular Disease involves the heart or blood vessels which include coronary artery disease, CVS include Heart Failure, Rheumatic Heart Disease, Abnormal Heart Rhythm. Approximately 17.9 million people died due to CVD in year 2016. The proper study of the heart, the mechanism of pathogenesis the animal model of cardiovascular disease is approach, for that the animal model which satisfy the need of regeneration of heart, the zebrafish have the property of regeneration of heart through its lifespan. From among animals the zebrafish is on priority for regeneration of heart. 16,17
Heart regeneration in Zebrafish
The zebrafish have the regenerative properties it can regenerate many organ & tissues such as Retinal, Brain tissues, Fines, Spinal cord & cardiac muscles. Like neonatal mice is also having the capacity for regeneration of the heart, but this property lost on the seventh day of birth
The zebrafish contain the exceptional capacity of regeneration of heart after causing any ventricular injury, the blood starts clothing at the site of injury to stop the flow of blood, The platelets plug is formed at the site of injury which contains Thrombin receptor on the surface which binds to Thrombin molecule which is converted into the fibrinogen solution into Fibrin as the fibrin have the property of forming long strand of insoluble protein which cover the platelets. Fibrin Stabilizing Factor helps in harden and contract, further which is form cardiac muscle, it takes around 1-2 month for total regeneration of the heart.18,19
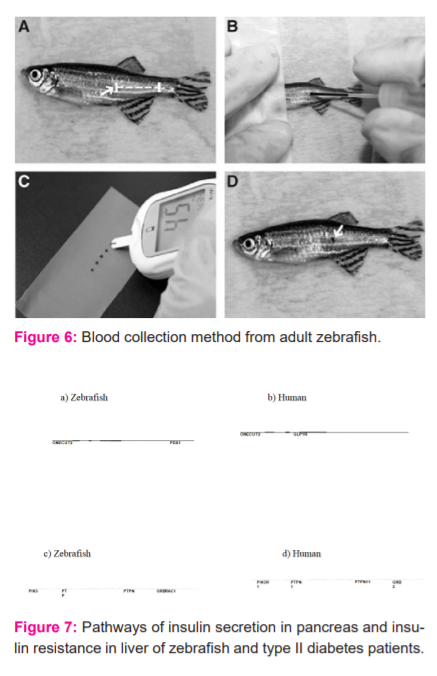
Cryoinjury is an alternative method for the injury of the muscles that helps in proper image analysis through the Olympus Microscope. Cryoinjury is the death of cell and tissues due to the lowering of temperature. In this method of Cryoinjury, the fish is anaesthetized using tricaine solution (0.032% wt/vol) in freshwater after that the anaesthetized fish is placed on the foam for detection in the microscope, it should be the focus on the level of heart which is in between the operculum and the base of the fin, then with the help of forceps hold the body of fish and with the help microdissection scissors make a small ventricular cut. The apex and ventral part of the ventricle must be visible, the excess of water is removing by blotting with tissue then apply precooled cryoprobe to the ventricular surface to quickly freeze the portion. Figure 5 interpret the Blood collection method from adult zebrafish. (A) Zebrafish (adult) anesthetised. (B) Blood is collected by inserting the needle at the blood collection site and then gently the blood is collected. (C) Blood is expelled from the needle onto a clean piece of parafilm. (D) Afterword’s the puncture site after blood collection is complete.20,21
It should be stick to the ventricular surface and the heartbeat gets slower gradually for a particular period after that put the fish into the freshwater, the water is blown through the pipette near the gills, the movement of gills will start within 4-5 minutes and start swimming within 8-10 minutes. After the period the zebrafish is again dissected by making an incision to reach toward the heart, the heart is washed with FDB sol and fixed with 4% (wt/vol) overnight for imaging by comparing the image the regeneration of heart is seen.22

C) Zebrafish Model for Type 2 Diabetes Mellitus
In Type-II Diabetes Mellitus the body doesn’t produce enough insulin and no loss of β-cell mass. Around 90% of the patient is of type 2 Diabetes Mellitus. The main cause is an abnormality in glucose receptor of β-cell so that they respond to the higher glucose concentration and reduce the sensitivity of peripheral tissue to insulin. In experimental animal, diabetes can be induced by overfeeding the glucose for around 3 months and the change in the concentration of glucose in the blood can be seen through the different test.23
Methods
Glucose tolerance test:
In this type two tolerance test is performed
A) Intraperitoneal glucose tolerance test i.e. (IPGTT)
B) Oral glucose tolerance test i.e. (OGTT)
Intraperitoneal glucose tolerance test in this test the fish were anaesthetized using ice water for around 5-minutes. 0.5 mg/g fish weight were injected Intraperitoneally and allow to recover after period interval 30, 90, 180 the blood is withdrawn through the proper instrument and blood glucose were determined at each time interval of 30, 90, 180.24,25
Oral glucose tolerance test in this fish is first anaesthetized, micropipette with a small tip is used for the administration of glucose through the mouth at dose 1.25 mg/g fish weight and allow to recover for 30, 90, 180-time interval and that particular time interval the blood sample were collected and blood glucose level is determined. Figure 6 shows the blood collection method from adult zebrafish for interpretation of glucose level.
Ins-EGFP image analysis
Enhance Green Fluorescent Protein the zebrafish is overfeeding through a micropipette for 3 months and the night before the testing the experimental animal is kept fast and anaesthetize in a tank containing 500 ppm of 2-phenoxyethanol after anaesthetizing the experimental animal it is kept under the Olympus SZX7 microscope with Green Fluorescence Protein (GFP) filter. The image obtained is quantified in ImageJ software.
The image is import and converts into a greyscale of red green blue( RGB). The RGB signal is split and the green signal is extracted, from that intensity of green fluorescent intensity from the pancreatic area is measure using pixel density. The intensity of fluorescence was converted into a percentage, the relative intensity of normal feed zebrafish is calculated for normal reading.26,27
Metformin and glimepiride administration
Metformin & Glimepridin are the drug use for the control of high blood glucose. In type-2 diabetes the administration of metformin to Zebrafish through proper concentration of 20 µM the concentration should be change daily. The blood sample was collected after 7 days of dose. Glimepiride is dissolved in dimethyl sulfoxide and make 500mm stock, dilute with fresh water up to 100 µM after 24 hr the blood sample is collected and tested.28,29
Figure 7 shows the Pathways of insulin secretion in the pancreas and insulin resistance in the liver of zebrafish and type II diabetes patients. Insulin secretion pathways in (a) zebrafish and (b) pancreatic beta cells of type II diabetes human patients. Pathways of insulin resistance in (c) zebrafish and (d) human hyperglycaemic liver. In this figure, red and blue denote genes with increased and decreased expression, respectively. Grey denotes genes that were not detected in the RNA-sea assay or DNA microarray technique.
CONCLUSION
As per the 3R principle i.e reducing waste, reusing and recycling resources and products, choosing to use things with care. Therefore Zebrafish can replace the most of the common animal model of various behavioural screening like a rat, mice, hamster as per the committee for control and supervision of experiments on animals (CPCSEA). According to CPCSEA zebrafish is more economical, easy to maintain and shows more convincing result than listed experimental animals. So it stands as an emerging model for pharmacological behaviour screening.
CONFLICT OF INTEREST: None
SOURCE OF FUNDING: None
ACKNOWLEDGEMENT: Nil
References:
1. Baker K, Warren K, Yellen G, Fishman M. Defective ‘‘pacemaker’’ current (Ih) in a zebrafish mutant with a slow heart rate. Proc Natl Acad Sci. 1997;94(6):4554–4559.
2. Barbazuk W, Korf I, Kadavi C, Heyen J, Tate S, Wun E, et al. The syntenic relationship of the zebrafish and human genomes. Genome Res. 2000;10(8):1351–1358.
3. Becker T, Wulliman M, Becker C, Bernhardt R, Schachner M. Axonal regrowth after spinal cord transection in adult zebrafish. J Comp Neurol. 1997; 377:577–595.
4. Beis D, Stainier D. In vivo cell biology: following the zebrafish trend. Trends Cell Biol.2006; 16 (10):105–112.
5. Boselli F, Vermont J. Live imaging and modelling for shear stress quantification in the embryonic zebrafish heart. Trends Cell Biol. 2010;12(8):105–112.
6. San Chi N, Shaw R, Jungblut B, Huisken J, Ferrer T. Genetic and physiologic dissection of the vertebrate cardiac conduction system. PLoS Biol. 2010; 6(5): e109.
7. Curado S, Anderson R, Jungblut B, Mumm J, Schroeter E, Stainier D. Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev Dyn 2007; 236:1025–1035
8. Fisher S, Grice E, Vinton R, Bessling S, McCallion A. Conservation of RET regulatory function from human to zebrafish without sequence similarity. Science 2006;3(12):276–279.
9. Chablais F, Veit J, Rainer G, Jaz´win´ska A. The zebrafish heart regenerates after cryoinjury-induced myocardial infarction. BMC Dev Biol. 2011;11(4):21-25.
10. Frangogiannis N. The mechanistic basis of infarct healing. Antio Red Signal. 2006; 8(4):1907–1939.
11. Gonza´lez-Rosa J, Mart V, Peralta M, Torres M, Mercader N. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. BMC Dev Biol. 2006;138:1663–1674.
12. Haack T, Abdelilah-Seyfried S. The force within endocardial development, mechanotransduction and signalling during cardiac morphogenesis. BMC Dev Biol. 2016; 143:373–386.
13. Jopling C, Sleep E, Raya M, Mart?´ M, Belmonte I. Zebrafish heart regeneration occurs by cardiomyocyte de-differentiation and proliferation. Nature 2010; 464:606–609.
14. Kalogirou S, Malissovas N, Moro E, Argenton F, Stainier D, Beis D. Intra-cardiac flow dynamics regulate atrioventricular valve morphogenesis. Cardi Res. 2014;104:49–60.
15. Kikuchi K, Holdway J, Major R, Blum N, Dahn R. Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev Cell 2011; 20:397–404.
16. Kikuchi K, Holdway J, Werdich A, Anderson R, Fang Y. Primary contribution to zebrafish heart regeneration by gata cardiomyocytes. Nature 2010;464:601–605.
17. Kim J, Wu Q, Zhang Y, Wiens K, Huang Y. PDGF signalling is required for epicardial function and blood vessel formation in regenerating zebrafish hearts. Proc Natl Acad Sci. 2010;107:17206–17210.
18. Kroehne V, Freudenreich D, Hans S, Kaslin J, Brand M. Regeneration of the adult zebrafish brain from neurogenic radialglia- type progenitors. Dev Acad Sci 2011;138:4831–4841.
19. Lam S, Wu Y, Vega V, Miller L, Spitsbergen J. Conservation of gene expression signatures between zebrafish and human liver tumours and tumour progression. Nat Biotechnol 2005;24:73–75.
20. Langheinrich U, Vacuum G, Wagner T. Zebrafifish embryos express an orthologue of HERG and are sensitive toward a range of QT-prolonging drugs inducing severe arrhythmia. Toxicol Appl Pharmacol 2003;193:370–382.
21. Kroehne V, Freudenreich D, Hans S, Kaslin J, Brand M. Regeneration of the adult zebrafifish brain from neurogenic radial glia-type progenitors. Dev Acad Sci. 2011;138:4831–4841.
22. Lam S, Wu Y, Vega V, Miller L, Spitsbergen J. Conservation of gene expression signatures between zebrafish and human liver tumours and tumour progression. Nat Biotechnol 2005; 24:73–75.
23. Langheinrich U, Vacuum G, Wagner T. Zebrafish embryos express an orthologue of HERG and are sensitive toward a range of QT-prolonging drugs inducing severe arrhythmia. Toxicol Appl Pharmacol 2003;193:370–382.
24. Milan D, Peterson T, Ruskin J, Peterson R, MacRae C. Drugs that induce repolarization abnormalities to cause bradycardia in zebrafish. Circulation 2003;107:1355–1358.
25. Porrello E, Mahmoud A, Simpson E, Hill J, Richardson J, Olson E. Transient regenerative potential of the neonatal mouse heart. Science 2011; 331:1078–1080.
26. Poss K, Keating M, Nechiporuk A. Tales of regeneration in zebrafish. Dev Dyn 2003; 22(6):202–210.
27. Smith KA, Joziasse IC, Chocron S, van Dinther M, Guryev V. Dominantnegative ALK2 allele associates with congenital heart defects. Circulation 2003; 11(9):3062–3069
28. Sehnert A, Huq A, Weinstein B, Walker C. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat Genet 2002; 31:106–110.
29. Steed E, Boselli F, Vermont J. Hemodynamics has driven cardiac valve morphogenesis. Biochim Biophys Acta 2015;7 (17 B):1760–1766.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License