IJCRR - 13(11), June, 2021
Pages: 49-52
Date of Publication: 04-Jun-2021
Print Article
Download XML Download PDF
Coexistent Xanthogranulomatous Cholecystitis and Carcinoma Gall Bladder: A Diagnostic Dilemma: One Year Study at a Tertiary Care Centre
Author: Aiffa Aiman, Zubaida Rasool, Nuzhat Samoon, Duree Mateen, Meesa Zargar, Rukhsana Akhtar, Mir Yasir
Category: Healthcare
Abstract:Introduction: Xanthogranulomatous cholecystitis is an uncommon variant of chronic cholecystitis that can present with marked gall bladder thickening or as a mass lesion mimicking a malignant neoplasm. Rarely it may be associated with carcinoma gall bladder, hence a thorough sampling is needed to exclude carcinoma gall bladder. Objective: To analyse the coexistence of xanthogranulomatous cholecystitis and carcinoma gall bladder. Methods: A one-year study was carried out in the department of pathology, from 1st November 2018 to 31st October 2019; in which the carcinoma gall bladder specimens were analysed grossly and microscopically and an association of carcinoma gall bladder with xanthogranulomatous cholecystitis was analysed. Results: A total of 30 cases of carcinoma gall bladder were reported of which 3 cases showed coexisting xanthogranulomatous cholecystitis. Conclusion: The coexistence of xanthogranulomatous cholecystitis and carcinoma of the gallbladder may present a diagnostic dilemma. Due to the overlapping clinical, radiological and gross findings. Although rare the possibility of a coexisting tumour needs to be excluded, for which immunohistochemical markers may be done.
Keywords: Adenocarcinoma gall bladder, Xanthogranulomatous cholecystitis, Carcinoma gall bladder, Granulomatous inflammation, Perineural invasion, Giant cells
Full Text:
Introduction
Xanthogranulomatous cholecystitis (XGC) is an unusual focal or diffuse destructive inflammatory process of the gall bladder, representing between 0.7 and 13.2% of all gallbladder diseases.¹ Christensen and Ishak were among the first to describe this entity as a pseudotumor of the gallbladder (fibroxanthogranulomatous cholecystitis) with an unusual, destructive type of inflammation, desmoplasia, pericholecystic infiltration and hepatic involvement.2 It is used to describe the lesion which results when lipids from the bile in the lumen of the gall bladder enter the wall of the organ and induce a granulomatous inflammation.3 The malignant potential of XGC is controversial and the relationship between XGC and gall bladder carcinoma (GBC) is unclear. Simultaneous XGC and GBC have been reported in some series with incidences ranging from 2% to 7.5%respectively.4,5 While others has reported the incidence between 0.2% to 35.4% of cases.6-8
MaterialS and MethodS
A one-year study was carried out in our department from 1st November 2018 to 31st October 2019, in which the carcinoma gall bladder specimens were analysed grossly and microscopically and an association of carcinoma gall bladder with xanthogranulomatous cholecystitis was seen. A total of 30 cases of carcinoma gall bladder were seen of which 3 cases showed coexisting xanthogranulomatous cholecystitis.
Case 1: A 55-year-old female presented with pain upper abdomen radiating to the back along with jaundice. USG was suggestive of a gall bladder mass. CECT was done which showed an asymmetric enhancing mural thickening involving the body of the gall bladder. LFT depicted hyperbilirubinemia with serum bilirubin of 5.5mg/dl. Intraoperatively a solid mass was seen in the body and fundus of the gall bladder not infiltrating the adjacent liver.
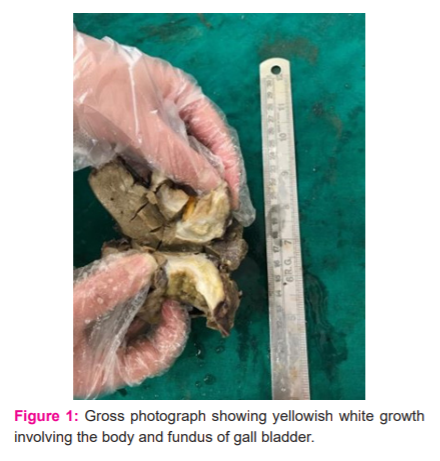
On gross examination, the resected gall bladder measured 9x2.5 cm with external surface haemorrhagic and roughened. On C/S grey-white to yellow growth identified measuring 3x2.5 cm. The growth was 1.5 cms from the surgical cut end (Figure 1).
Microscopically a well-differentiated adenocarcinoma was seen infiltrating into the perimuscular connective tissue (pT2). The background showed aggregates of foamy macrophages with occasional foreign body giant cells, few spindles shaped elongated macrophages, plasma cells and lymphocytes, suggestive of xanthogranulomatous cholecystitis (Figure 2).
Case 2: A 50-year-old male presented with pain upper abdomen. USG showed a mass in the body of the gall bladder. CECT showed an eccentric circumferential enhancing thickening in the mid-body of the gall bladder. Intraoperatively a thickened firm polypoidal mass was seen involving the fundus and body of the gall bladder. Serum bilirubin was 2.8 mg/dl.
Gross examination of the resected specimen showed a polypoidal growth measuring 2x2 cm in the body of the gall bladder. C/S through the growth was grey-white infiltrating into the muscularis. The growth was 4 cm from the gall bladder and 5 cm from the liver resection margin. Serial sections through the attached liver tissue were unremarkable.
Microscopy revealed a moderately differentiated adenocarcinoma limited to the gall bladder wall however going beyond muscularis propria (pT2). The gall bladder resection margin showed high-grade dysplasia and the hepatic resection margin was free of tumour. Lymphovascular invasion was seen, however, there was no perineural invasion. Few sections from the growth revealed large round macrophages with pale granular cytoplasm, foreign body giant cells and chronic inflammatory cell infiltrate which were indicative of a background of xanthogranulomatous cholecystitis. Immunostaining for CK 7 was positive in the malignant glands (Figure 3).
Case 3: A 60-year-old male presented with a one-week history of jaundice, anorexia and upper abdominal pain. Serum bilirubin was 6.7 mg/dl. CECT showed a circumferentially enhancing growth in the fundus and another growth in the mid-CBD along with cholelithiasis. Intraoperatively a growth was identified in the fundus of the gall bladder and another growth in the gall bladder neck.
On gross examination, the fundus of the gall bladder showed a friable yellow-brown area and the neck of the gall bladder showed a grey-white circumferential growth. In addition, multiple calculi were identified in the gall bladder.
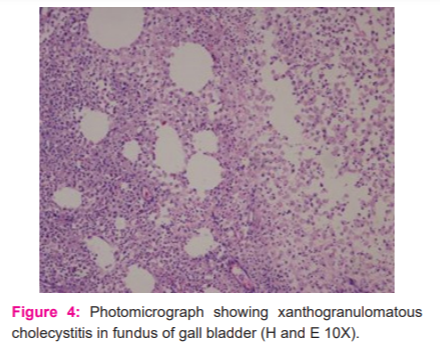
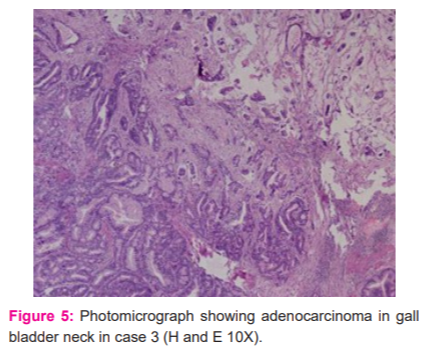
Microscopically sections from the fundus showed features of xanthogranulomatous cholecystitis while those from the neck showed moderately differentiated adenocarcinoma limited to the wall of the gall bladder (pT1) (Figures 4 and 5)
Results and Discussion
In this study, we describe the clinicopathological features of coexisting xanthogranulomatous cholecystitis and adenocarcinoma of the gall bladder. Xanthogranulomatous cholecystitis is an uncommon form of chronic cholecystitis characterized by a focal or diffuse destructive inflammatory process, with varying proportions of fibrous tissue, acute and chronic inflammatory cells and accumulation of lipid-laden macrophages in areas of inflammation.1
The pathogenesis of this lesion is not well understood, although it is believed that a rupture of the Rokitansky-Aschoff sinuses with extravasation of bile in the interstitial tissues and consequent xanthogranulomatous inflammatory reaction is the initial causes.9,10,11 This theory is supported by the frequent finding of bile and mucus in the lesion, and by the occasional finding of a focus of XGC with a disrupted sinus.2,3,10 Gallbladder carcinoma might provide a route for bile to enter the stroma more readily than in chronic cholecystitis or cholelithiasis because of the greater tissue destruction associated with it, thus explaining the coexistence of these two conditions.12
Xanthogranulomatous cholecystitis often mimics a gallbladder carcinoma, leading to a diagnostic dilemma. XGC distorts the outline of the gallbladder, forms adhesions with adjacent tissues, and participates in fistula formation.3 This may cause misdiagnosis as gallbladder carcinoma on preoperative evaluation and at laparotomy; in particular, XGC may look like a malignant neoplasm on ultrasonography.13,14
Preoperatively and intraoperatively, it is difficult to diagnose this entity and the final diagnosis is usually based on histological examination of the resected specimen. The malignant potential of XGC is controversial and highly disputed.1,6,7,8 The association of XGC and GBC is a matter of discussion. According to some authors, it may simply be that XGC and adenocarcinoma are both complications of cholelithiasis and cholecystitis of a particular duration or degree, or that tissue disruption by a carcinoma facilitates the entry of bile into the stroma.12
The association between XGC and gallbladder cancer has been shown in the literature in small case series and some single case reports. An Indian study reported that only 0.2% of patients with XGC have associated GBC.6 On the other hand, is an American study, among 40 cases of XGC, five [12.5%] also had GBC.10 In the United Kingdom, a study of 31 patients with XGC revealed carcinoma in three [9.7%] patients.8 In a Japanese study, 10% of XGC patients also had GBC.1 In a Mexican study, XGC was associated with carcinoma in five cases (3%) among a total of 182 cases of XGC. According to the authors of that study, the inflammatory reaction followed by an associated immunologic cellular response may produce the appearance of cellular changes that degenerate into carcinoma.1 In our study we encountered 3 cases of coexisting XGC and carcinoma gallbladder out of 30 cases of GBC in one year accounting for an incidence of 10%. This correlates most with the Japanese study.
The possible association of XGC and GBC carries a risk of diagnostic confusion. Pathologists might fail to notice the presence of GBC when it is associated with florid XGC. Furthermore, the existence of florid XGC may lead to errors in determining the exact stage of the malignant spread of GBC as macrophages can be confused with tumour cells.12
Conclusion
The coexistence of XGC and carcinoma of the gallbladder may present a diagnostic dilemma. Due to the overlapping clinical, radiological and gross findings of xanthogranulomatous cholecystitis and carcinoma of the gall bladder, Definitive diagnosis of XGC relies on extensive sampling and thorough microscopic examination of the surgical specimen. Although rare, the possibility of a coexisting tumour needs to be excluded, for which immune-histochemical markers like CD 68 for xanthomatous cells and CK 7 for neoplastic glands may also be used in addition to routine H&E staining.
Acknowledgement: Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors/editors/publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
Conflict of Interest: Nil
Source of funding: Nil
Authors Contribution: Aiffa Aiman conceived the idea and wrote the manuscript with support from Nuzhat Samoon and Duree Mateen. Zubaida Rasool helped supervise the project. Meesa Zargar and Rukhsana Akhtar assisted with the photography. Mir Yasir helped in data compilation and correspondence with the journal.

References:
-
Guzman-Valdivia G. Xanthogranulomatous Cholecystitis: 15Years’ Experience. World J Surg 2004;28:254-7.
-
Christensen AH, Ishak KG. Benign tumours and pseudotumors of the gall bladder. Report of 180 cases. Arch Pathol 1970;90:423-32.
-
Roberts KM, Parsons MA. Xanthogranulomatous cholecystitis: a clinicopathological study of 13 cases. J Clin Pathol 1987;40:412-417.
-
Parra JA, Acinas O, Bueno J, Guezmes A, Fernandez MA, Farinas MC. Xanthogranulomatous cholecystitis: Clinical, sonographic, and CT findings in 26 patients. Am J Roentgenol 2000;174:979-83.
-
Solanki RL, Arora HL, Gaur SK, Anand VK, Gupta R. Xanthogranulomatous cholecystitis (XGC): A clinicopathological study of 21 cases. Indian J Pathol Microbiol 1989;32:256-60.
-
Dixit VK, Prakash A, Gupta A, et al. Xanthogranulomatous cholecystitis. Dig Dis Sci 1998;43:940-2.
-
Lee HS, Joo KR, Kim DH, et al. A case of simultaneous xanthogranulomatous cholecystitis and carcinoma of the gallbladder. Korean J Intern Med 2003;18:53-6.
-
Houston JP, Collins MC, Cameron I, et al. Xanthogranulomatous cholecystitis. Br J Surg 1994;81:1030-9.
-
Roberts KM, Parsons MA. Xanthogranulomatous cholecystitis: a clinicopathological study of 13 cases. J Clin Pathol 1987; 40:412–417.
-
Goodman ZD, Ishak KG. Xanthogranulomatous cholecystitis. Am J Surg Pathol 1981;5:653–659.
-
Christensen AH, Ishak KG. Benign tumours and pseudotumors of the gallbladder. Arch Pathol Lab Med 90:423–4321970.
-
Benbow EW. Xanthogranulomatous cholecystitis associated with carcinoma of the gallbladder. Postgrad Med J 1989;65:528-31.
-
Bluth EI, Katz MM, Merritt CRB, Sullivan MA, Mitchell WT. Echographic findings in xanthogranulomatous cholecystitis. J Clin Ultrasound 1979;7:213-214.
-
Gockel HP. Xanthogranulomatose cholezystitis. Fortschr Rontgenstr 1984;140:223-224.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License