IJCRR - 13(10), May, 2021
Pages: 107-116
Date of Publication: 19-May-2021
Print Article
Download XML Download PDF
Development of Fenofibrate Solid Dispersions for the Plausible Aqueous Solubility Augmentation of this BCS Class-II Drug
Author: Purushottam S. Gangane, Vaibhav M. Mule, Debarshi Kar Mahapatra, Nilesh M. Mahajan, Harigopal S. Sawarkar
Category: Healthcare
Abstract:Background: Fenofibrate is having a broad spectrum lipid-lowering activity that produces substantial reductions in the fatty components. The drug belongs to BCS Class-II and therefore in the majority of the cases, low pharmacodynamic potentials have been perceived and it requires serious improvement in the aqueous solubility. For the marked solubility enhancement of drugs, solid dispersions are an impressive approach. Objective: The current research emphasized on development of fenofibrate solid dispersions by employing the polymer PEG 6000 in the ratio of 1:1 w/w - 1:3 w/w using the fusion method/melting method/solvent evaporation method for enhancing the aqueous solubility that will directly affect the dissolution process and ultimately the therapeutic bioavailability. Methods: The fabricated solid dispersions were characterized for production yield, drug content, bulk density, tapped density, Carr's index, Hausner's ratio, angle of repose, and cumulative drug release. The transformation from crystalline nature to the amorphous form was characterized by DSC and XRD techniques. The optimized solid dispersion formulations were further loaded into the capsule and the content uniformity, drug content, disintegration time, and cumulative drug release in both the medium for 1 hr duration were investigated. Results: The physical mixtures exhibited the lowest drug release in the range 15%-26% at increasing polymeric ratios while the highest drug release was observed in solid dispersion batch prepared by fusion method (22.56%-34.89%). FT-IR studies indicated no incompatibilities between the drug and the polymer. Conclusion: This interesting investigation opened new avenues for the pharmacotherapeutic perspective of this drug in the upcoming future, both pharmacodynamically and pharmacokinetically by enhancing the aqueous solubility
Keywords: Fenofibrate, Solid dispersion, Solubility, Bioavailability, Dissolution, Enhancement
Full Text:
INTRODUCTION
Fenofibrate is a fabric acid derivative having a broad spectrum lipid-lowering activity (lipid-modifying effects) that is primarily mediated by the activation of the therapeutic target - peroxisome proliferator-activated receptor-α (PPAR-α).1 This privileged drug produces substantial reductions in the plasma triglyceride level and low-density lipoprotein (LDL) level while increasing the levels of high-density lipoprotein (HDL).2 It acts to endorse the clearance of chylomicrons and very-low-density lipoproteins (VLDL) and can improvise the irregularities associated with the LDL subfractions with a transfer from dense LDL.3 The drug is principally recommended in improving the lipoprotein atherogenic phenotypes in patients suffering from coronary heart disease (CHD).4 Fenofibrate also possesses pleiotropic potentials such as reducing the levels of pro-inflammatory factors, C-reactive protein, fibrinogen, and simultaneously improves the microvascular outcomes as well as flow-mediated dilatation that results in clinical effectiveness.5 It is broadly absorbed exclusively in the presence of food and the presence of albumin, it is quickly transported through the human bloodstream.6 In normal persons, at a steady-state with customary doses of 100 mg t.i.d., it initiates a series of episodes that result in the lessening of apolipoprotein C-III formation in the human liver with a long plasma half-life of ~30 hrs.7 Fenofibrate is taken up by both the key organs of the human body; liver and kidney. Apart from a small percentage (~5%) reduction before the conjugation process at the ketone moiety, in the human urine, the majority of the drug is excreted as a conjugate. <20% is excreted through human bile.8 The drug belongs to Biopharmaceutics Classification System (BCS) Class-II and therefore has high permeability, low solubility characteristics.9 Therefore, in the majority of the cases, low pharmacodynamic potentials have been perceived and it requires serious improvement in the aqueous solubility.
Among the majority of capable approaches for the possible enhancement of drug solubility and associated dissolution of hydrophobic drug molecules (particularly for BCS Class-II and Class-IV drugs), solid dispersion is a promising way where the drug molecules are dispersed in the inert carrier (such as a polymer, etc.).10 The drug through reduced morphological features, providing high surface area, existence into an amorphous form from former crystallinity, and absolute drug encapsulation within the polymer matrix exhibit drastic improvement in solubility profile and swiftly enhances the dissolution rates.11 When solid dispersion is formulated, energy is not required in breaking the drug crystalline lattice, thereby promoting the improvement of the dissolution process (facilitating better drug wettability) which results in a better release and leads to high bioavailability in comparison to the conventional formulations.12
The current research emphasized on development of fenofibrate solid dispersions by employing the polymer PEG 6000 in the ratio of 1:1 w/w, 1:2 w/w, and 1:3 w/w using the fusion method/melting method and solvent evaporation method for enhancing the aqueous solubility that will directly affect the dissolution process and ultimately the therapeutic bioavailability. The compatibility study was studied by Fourier transformed infrared spectroscopy. The fabricated solid dispersions were characterized for production yield, drug content, bulk density, tapped density, Carr’s index, Hausner’s ratio, angle of repose, and cumulative drug release (in 0.1% SLS medium and pH 6.8 phosphate buffer medium). The transformation from crystalline nature to the amorphous form was characterized by Differential scanning calorimetry (DSC) and X-ray diffraction (XRD) techniques. The optimized solid dispersion formulations (FM 1:3 and SE 1:3) were further loaded into the capsule according to the equivalent weight of 54 mg and the content uniformity, drug content, disintegration time, and cumulative drug release in both the medium for 1 hr duration were investigated.
MATERIALS AND METHODS
Materials
Pharmaceutical grade fenofibrate was obtained as a generous gift sample from Enaltech Pvt. Ltd., Mumbai, India. Loba Chem Ltd., Mumbai, India remained the chief supplier for analytical grade PEG 6000, methylene chloride, sodium lauryl sulfate (SLS), sodium dihydrogen phosphate, potassium dihydrogen phosphate, methanol, and ethanol. Double-distilled water (Borosil®, Mumbai, India) was employed during this research work.
Instruments
UV/Vis spectrophotometer (Shimadzu® UV-1800, Japan), Weighing balance (Wensar Weighing Scales Limited, India), Differential scanning calorimetry (Mettler Toledo®, USA), Sonicator (PCI analytical 100HPOTC, India), X-ray diffraction (Ultima-III, Rigaku®, Japan), Rotary shaker (Biotechniques® BIP05B, India), Fourier transform infrared spectroscopy (GX-FT-IR, Perkin Elmer®, USA), Hot air oven (Biotechniques® Limited, India), pH meter (Labtronics® LT-10, New Delhi, India), IP disintegration apparatus (Electrolab®, TDT 08L, Mumbai, India) and USP Dissolution 33 (Type-II) apparatus (Electrolab®, Mumbai, India) were employed for formulation and characterization of the solid dispersion formulations.
Drug and polymer interaction studies
The compatibility study between fenofibrate (pure drug) and PEG 6000 (polymer) was performed using a FT-IR spectrometer, before formulating the solid dispersions. The physical mixture of polymer-drug and developed solid dispersion formulations were also studied employing the KBr disks and scanned in the range of 4000 to 500 cm-1 to investigate the compatible nature of the drug with the polymer. The characteristic peaks were recorded at a resolution of 4 cm/s.13
Saturation solubility study
Fenofibrate, the drug molecule selected in this present research work has very poor aqueous solubility characteristics. For determining the aqueous solubility of fenofibrate with different ratios of PEG 6000 (polymer), the saturation solubility study was determined. The physical mixture of fenofibrate with PEG 6000 was added into glass-stoppered flasks containing 10 mL of double-distilled water of increasing concentrations of PEG 6000 in the physical mixture ratios of 1:0 w/w, 1:1 w/w, 1:2 w/w, and 1:3 w/w. The flasks were sealed and shaken on a rotary shaker at room temperature. After equilibration for 96 hrs, the solutions were filtered through Whatman filter paper No. 41. Then, the filtrates were estimated by UV-Vis spectroscopy at λmax 286 nm.14
Preparation of solid dispersions
Solvent evaporation method
The solid dispersions of fenofibrate were prepared by the solvent evaporation method using the polymer PEG 6000 in various w/w ratios (1:1 w/w, 1:2 w/w, and 1:3 w/w). The drug and polymer were dissolved in 5 parts of methylene chloride (dichloromethane) in a closed glass receiver. After the complete dissolution of the content, the solvent was evaporated under room temperature. The obtained solid dispersions were dried and subsequently pulverized by triturating in pestle-mortar and subsequently screened through #66 mesh sieve.15
Melt method/Fusion method
The solid dispersions of fenofibrate were prepared by the melt method/fusion method using the polymer PEG 6000 in various w/w ratios (1:1 w/w, 1:2 w/w, and 1:3 w/w). The polymer PEG 6000 was placed in a porcelain dish and allowed to melt by heating above the melting point of the carrier for the duration of 5 min with continuous stirring until a homogenous dispersion was obtained. The resultant solution was cooled in an ice bath for rapid solidification. The content was scrapped off, pulverized further, and finally passed through sieve #66.16
Characterization of solid dispersions
Production yield
The percentage of production yield was calculated to determine the % yield or efficiency of any method. Thus, it is helpful in the selection of an appropriate method of production. The produced solid dispersions were collected and weight to determine production yield from the formula17:
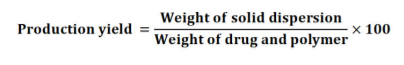
Drug content
Accurately weight prepared solid dispersions equivalent to 10 mg of fenofibrate was transferred to 10 mL volumetric flask containing 10 mL of methanol and the above content was suitably dissolved. 1 mL of this solution was diluted to 10 folds with methanol and the absorbance was measured at λmax 286 nm.18
Bulk density
It refers to the packing of particles. Bulk density was used to determine the amount of drug that occupies the volume in g/mL. The bulk density of the ingredients was evaluated using a graduated cylinder. It is the ratio of the total mass of powder to the bulk volume of powder. It was measured by pouring the weighed quantity of powder into a graduated measuring cylinder and the volume was noted. It was expressed in g/mL and is calculated by using the following formula19:
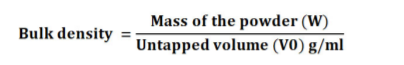
Tapped density
It is the ratio of the total mass of powder to the tapped volume of powder. The tapped volume was measured by tapping the powder 10, 500, and 1250 taps in tap density apparatus (Electro Lab USP II) according to USP. The blend was subjected for 500 taps; % volume variation was calculated and subjected for additional 1250 taps, and the % variation was calculated from the formula20:

Carr’s index
The compressibility index is an important measure that can be obtained from the bulk and tapped densities. In theory, the less the compressible material, the more flowable it is. A material having values of less than 20% is defined as a free-flowing material. The relationship between % compressibility indexes with flowability can be given by21:

Hausner’s ratio
It designates the granules flow characteristics and is estimated from the tapped density: bulk density ratio. It is determined from the formula22:
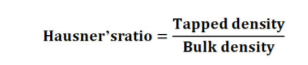
Angle of repose
The angle of repose is an indication of the friction forces existing among the granule components. It is the utmost angle likely among the face of the pile of the granules and the horizontal flat surface. The angle of repose was determined by bypassing the fixed quantity of powder from the funnel at constant height till the top of the pile made by the powder touches the funnel. The flowability of the granules was determined by calculating the angle of repose by fixed height method23:
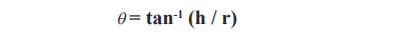
where, θ = angle of repose; h = height of pile; r = average radius of powder cone.
= angle of repose; h = height of pile; r = average radius of powder cone.
Thermal characteristics
By using the differential scanning calorimeter, the thermogram of the pure drug (fenofibrate), polymer (PEG 6000), physical mixture, and optimized microsphere formulations (FM 1:3 and SE 1:3) were recorded by heating in the range of 30-300°C at a heating rate of 10°C/min to study the thermal behaviour. The heating was performed under an inert nitrogen atmosphere (20 mL/min flushing).24
Physical state examination
By using the X-ray diffractometer, the diffraction patterns of the pure drug (fenofibrate), polymer (PEG 6000), physical mixture, and optimized microsphere formulations (FM 1:3 and SE 1:3) were recorded by irradiating the samples with Cu-Kα monochromatized radiation (40 kV, 35 mA) in the 2θ range of 3-60° to study the physical state.25
In vitro dissolution studies
The in vitro dissolution characteristics of the fabricated solid dispersions were studied by utilizing the paddle-type dissolution test apparatus in 0.1% sodium lauryl sulfate containing 900 mL simulated gastric fluid media without any enzymes, maintained at a temperature of 37°C ± 0.5°C at a stirring speed of 50 rpm. The solid dispersions equivalent to 500 mg of fenofibrate were placed in the dissolution medium. At a specific interval of time (0 min to 60 min), 1 ml of sample was withdrawn from each vessel, filtered through Whatman filter paper No.41, diluted analytically, and analyzed spectrophotometrically at λmax 286 nm. After every sampling, equivolume pre-warmed fresh dissolution medium was replenished to the system to retain the steady volume throughout the experiment. The cumulative releases from the solid dispersion formulations were performed in a triplicate manner and the study was expressed in percentage.26
Formulation of fenofibrate solid dispersion loaded capsule dosage form
Accurately weighted, optimized solid dispersions; FM 1:3 (158 mg) and SE 1:3 (149 mg), equivalent to 54 mg were filled into the hard gelatin capsule without excipients. 50 capsules were made and further evaluated for weight uniformity, drug content, disintegration studies, and dissolution studies.
Evaluation of fenofibrate solid dispersion loaded capsule dosage form
Weight uniformity
20 capsules were randomly selected from each batch and individually weighed. The average weight and standard deviation of 20 capsules were calculated. The batch passes the test for weight variation if NMT 2 of the individual capsule weight deviates from the average weight as shown in the official USP monographs and none of them deviates by more than twice the percentage shown in the capsule. For the content uniformity test, the capsule should contain not less than 95% and not more than 105% of the labelled potency.19
Drug content
From the capsule, the optimized solid dispersions equivalent to 50 mg of fenofibrate was transferred to a 50 mL volumetric flask containing 50 mL of methanol and the above content was suitably dissolved. 1 mL of this solution was diluted to 10 folds with methanol and the absorbance was measured at λmax 286 nm. The results are expressed in % of the drug present in the capsule formulation.18
Disintegration study
The disintegration testing of the capsule was performed using the disintegration tester comprising of basket constitution holding plastic tubes, 6 in number, which was open from both sides and the bottom portion was covered with #10 mesh. The basket containing the capsule was immersed into 1 L of dissolution media (simulated gastric fluid containing 0.1% sodium lauryl sulfate) with 30 cycle mode (raised and lowered). The time in use for the complete disintegration of the capsule with no palpable mass remaining was taken into account and the time was recorded. The study was performed in a triplicate manner. For most capsules, the USP guidelines state that the product must disintegrate in the duration of 15 min.27
In vitro dissolution study
The in vitro dissolution characteristics of the capsules containing the optimized solid dispersion formulations (FM 1:3 and SE 1:3) were studied by utilizing the paddle-type dissolution test apparatus in 0.1% sodium lauryl sulfate containing 900 mL simulated gastric fluid media without any enzymes, maintained at a temperature of 37°C ± 0.5°C at a stirring speed of 50 rpm. The capsule was placed at the bottom of the paddle connected to a variable speed motor in the dissolution medium. At a specific interval of time (0 min to 60 min), 1 ml of sample was withdrawn from each vessel, filtered through Whatman filter paper No.41, diluted analytically, and analyzed spectrophotometrically at λmax 286 nm. After every sampling, equivolume pre-warmed fresh dissolution medium was replenished to the system to retain the steady volume throughout the experiment. The cumulative releases from the solid dispersion formulations were performed in a triplicate manner and the study was expressed in percentage.26
RESULTS AND DISCUSSION
Drug-polymer compatibility study
FT-IR spectra of the drug presented prominent peaks at 3433 cm-1, 2983 cm-1, 2563 cm-1, 1728 cm-1, 1651 cm-1, 1504 cm-1, 1419 cm-1, 1342 cm-1, 1249 cm-1, 1014 cm-1, 974 cm-1, 925 cm-1, 860 cm-1, 844 cm-1, 765 cm-1, 740 cm-1, 682 cm-1, 638 cm-1, and 624 cm-1, respectively (Figure 1). The polymer PEG 6000 demonstrated spectral peaks at 4332 cm-1, 4004 cm-1, 3703 cm-1, 2887 cm-1, 2694 cm-1, 2509 cm-1, 2357 cm-1, 2237 cm-1, 2166 cm-1, 1956 cm-1, 1728 cm-1, 1649 cm-1, 1598 cm-1, 1467 cm-1, 1411 cm-1, 1359 cm-1, 1342 cm-1, 1280 cm-1, 1242 cm-1, 1060 cm-1, 947 cm-1, 842 cm-1, 740 cm-1, and 528 cm-1, respectively. These peaks appeared nearly analogous in the physical mixture (drug + polymer) samples and solid dispersions produced through both the methods (FM and SE) in the ratio of 1:1 w/w, 1:2 w/w, and 1:3 w/w, which concluded that no prominent interaction of the drug with the polymer occurred while formulating the product.
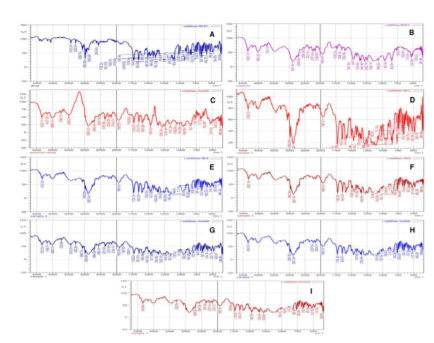
Figure 1. Drug-polymer interactions: (A) Fenofibrate pure drug; (B) PEG 6000 polymer; (C) Physical mixture (Drug + Polymer); (D) Solid dispersion produced by fusion method (1:1 ratio); (E) Solid dispersion produced by fusion method (1:2 ratio); (F) Solid dispersion produced by fusion method (1:3 ratio); (G) Solid dispersion produced by solvent evaporation (1:1 ratio); (H) Solid dispersion produced by solvent evaporation (1:2 ratio); and (I) Solid dispersion produced by solvent evaporation (1:3 ratio).
Saturation solubility study
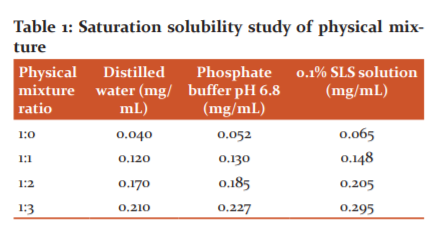
The pure drug fenofibrate demonstrated poor aqueous solubility of 0.040 mg/mL, however, the solubility of the drug augments (0.065 mg/mL) in the presence of 0.1% SLS due to the micellar effect which increases the solubilization (Table 1). The solubility of the drug enhances to 0.052 mg/mL in phosphate buffer pH 6.8 due to ionic interactions or miscellaneous ways. When hydrophilic structured vehicle PEG 6000 was added to the drug in equal quantity, the solubility of the drug enhances (0.120 mg/mL) rapidly as the drug gets dispersed in the carrier where reduced morphological features, high surface area, and existence into amorphous form results in augmentation of solubility which results in dissolution rate that in turn leads to increase in therapeutic bioavailability. When the polymer concentration was amplified to 3 times, the solubility of the drug boosted concurrently (0.210 mg/mL) due to better dispersion of the drug into the polymeric matrix which facilitated better solubility. When the solubility of the physical mixture with the highest polymer ratio was studied in the presence of 0.1% SLS, the solubility further improved to 0.295 mg/mL due to the surfactant effect which promotes rapid solubilization of the drug. A similar escalation of solubility (0.227 mg/mL) of the physical mixture with the highest polymer ratio was perceived owing to the ionic effect of the buffer solution.
Characterization of solid dispersions
The production yield was found to be in the range of 82.86% to 89.41% for solid dispersions produced from the fusion method while the case of solid dispersions produced from the solvent evaporation method showed a production yield in the range of 86.95% to 91.66% (Table 2). From the above observations, it can be concluded that the solvent evaporation method produced better yield as compared to the fusion method in the fabrication of solid dispersions. The production yield enhances with an increase in the concentration of hydrophilic polymer PEG 6000 which can be explained by better dispersion of the drug in the matrix of the polymer.
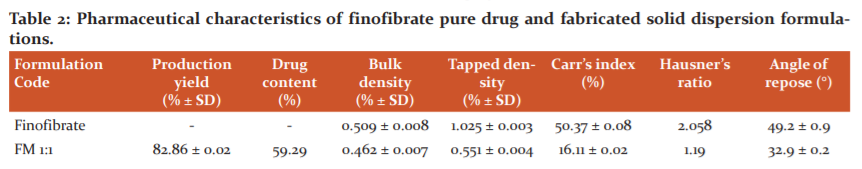
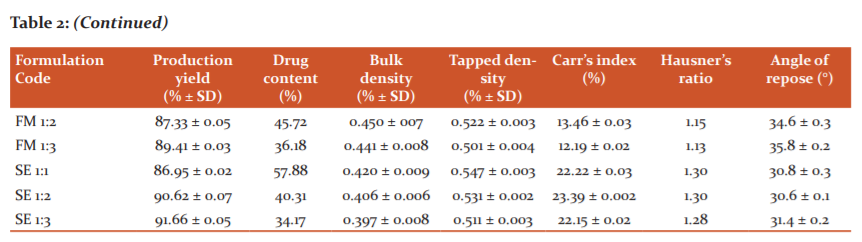
The drug contents in the developed formulations were found to be in the range of 36.18% to 59.29% for FM batch and 34.17% to 57.88% for SE batch (Table 2). The drug content was seen to decrease with an increase in the polymer concentration which may be due to reduced dispersion of the free drug in the matrix. However, several other factors may contribute towards the reduction of the drug content with the enhancement in the polymeric ratios. The ratio of 1:1 was found to be quite privileged while the highest concentration showed the lowest drug content.
The bulk density and tapped density lies in the range of 0.441% to 0.462% and 0.501% to 0.551%, respectively in the case of FM batches whereas 0.397% to 0.420% and 0.511% to 0.547%, respectively remained the observational range for the SE batch which represented excellent packing of the granules. The decrease in the bulk density and tapped density with the increase in the amount of polymer concentration was primarily observed which may be explained by the fact that the enhanced concentration of the hydrophilic structured vehicles provides compactness to the system by dispersing the drug uniformly into the matrix. Carr’s index and Hausner’s ratio were observed in the range of 12.19 to 16.11 and 1.13 to 1.19, respectively for solid dispersions of batch FM whereas the investigated values in the case of batch SE fall in the range of 22.15 to 23.39 and 1.28 to 1.30, respectively which indicated good flowability in case of former batch and fair flowability in latter batch. The angle of repose was found to be in the observational range of 32.9° to 35.8° for fusion batches whereas 30.8° to 31.4° for the solvent evaporated batches which represented good flow property. An augmentation in the angle of repose was observed when the polymer concentration was enhanced simultaneously because, with the increase in the polymeric bulk, the degree of compactness of the solid dispersion system gets comprised. However, when all the above parameters (densities and indexes) were compared with the free pure drug, a drastic improvement in the micromeritic properties has been observed. The correlation between polymer concentration and degree of compactness was found to play a critical role in the pharmaceutical characteristics of the fabricated product.
The thermal analysis of the pure drug, polymer, and solid dispersions were comprehensively studied by using differential scanning calorimetry (DSC). Fenofibrate, the drug presented a sharp endothermic peak corresponding to its melting point at 79.85°C whereas the polymer PEG 6000 showed a nearly sharp endotherm at 61.46°C. On analyzing the physical mixture (drug + polymer), it was observed that the endothermic peak shifted towards a lower value at 59.92°C which indicated keys steps such as reduced morphological features, high surface area, existence into amorphous form, and absolute drug encapsulation within the polymer matrix, which play the dominant role in the physical nature of the system. The analysis of the optimized solid dispersions (FM 1:3 and SE 1:3) produced by both the methods represents endothermic peak at 58.63°C and 57.60°C, respectively (Figure 2), which may be due to the alteration in the melting characteristics of the drug and inhibiting the crystalline nature as the amorphous state is believed to be a high disorder state where the solid particles stay.
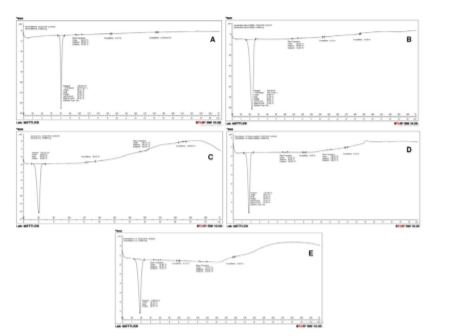
Figure 2. DSC thermograms: (A) Fenofibrate pure drug; (B) PEG 6000 polymer; (C) Physical mixture (Drug + Polymer); (D) Optimized solid dispersion formulation produced by fusion method; and (E) Optimized solid dispersion formulation produced by solvent evaporation method.
The physical state of the pure drug, polymer, and solid dispersions were comprehensively studied by using X-ray diffraction (XRD). The pure drug fenofibrate and polymer PEG 6000 showed several high peak intensities in the region 6-27° and 9-26° of 2θ which indicated the crystalline nature of the chemicals (Figure 3). While analyzing the physical nature of the solid dispersions produced by both the techniques (FM 1:3 and SE 1:3), it was observed that the drug remained in the molecularly dispersed state where the transformation of the drug from crystalline form to amorphous form occurs. This amorphous form has a very high internal energy that facilitates effective drug solubilization, promotes drug dissolution, and enhances drug bioavailability.
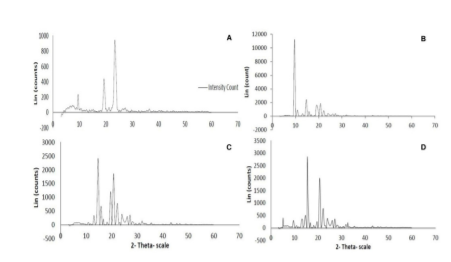
Figure 3. PXR Diffractograms: (A) Fenofibrate pure drug; (B) PEG 6000 polymer; (C) Optimized solid dispersion formulation produced by fusion method; (D) Optimized solid dispersion formulation produced by solvent evaporation method.
The prepared batches (physical mixture, solid dispersions prepared by fusion method, and solid dispersions produced by solvent evaporation method) expressed variable cumulative drug release in the range of 5.0% to 34.89%. The physical mixtures exhibited the lowest drug release in the range 15% to 26% at increasing polymeric ratios while the highest drug release was observed in solid dispersion batch prepared by fusion method (22.56% to 34.89%) in 0.1% SLS media (Table 3). When the two optimized formulations (FM 1:3 and SE 1:3) were studied in pH 6.8 phosphate buffer, a marginal decrease in the cumulative drug release was seen. It has been observed that the highest polymer ratio presented the highest drug release as compared to both the low and medium concentrations. The reason may be that the high polymeric system could tender additional accessible space for neighbouring hydrophobic drug particles which lead to swift hydration of the drug particles and produce enhanced wettability and augmentation in the dissolution process. The amorphous nature of the drug in the solid dispersions assists the advantaged drug release rate over the pure drug (5.0%). In the binary phase of the drug delivery system, the hydrophobic drug particles lie in contact with the hydrophilic polymer contents. When the drug and the polymer come in contact with the aqueous phase, hydration of the polymeric units occurs which transforms into the solution state, and ultimately leads to the drug solubilization and its release into the medium. In contrast to it, low polymer concentration leads to the formation of micelles (monomolecular) which converts into micelles (multi-molecular) on enhancing the concentration that facilitates greater solubilization of the drug by promoting better dissolution of the drug in the media.
Evaluation of fenofibrate solid dispersion loaded capsule
The weight variation of the formulated capsule batch was observed to be <1% which indicated almost uniform drug content in the formulations and a good pre-formulation and post-formulation characteristics. The accepted percentage deviation of ±7.5% was also taken into account where less than 324 mg weight was found to be applicable for the produced formulations.
The drug contents in the optimized solid dispersion loaded capsule formulations (FM 1:3 and SE 1:3) were found to be 97% to 96%, respectively (Table 4). The drug content was seen to be high due to better dispersion of the drug in the amorphous form in the polymeric matrix. Adequate quantity (nearer to 100%) of fenofibrate in the form of solid dispersion represented a sufficiently higher amount of readily soluble drug needed for delivery to the desired targets.
The disintegration time of the capsules containing the optimized solid dispersion (FM 1:3 and SE 1:3) was observed to be 7.31 min and 7.26 min, respectively. Both the capsule formulations demonstrated nearly equal disintegration characteristics owing to similar polymeric compositions. The capsules did comply with the USP guidelines which states that the product must disintegrate in 15 min.
The dissolution characteristics of both the solid dispersion containing formulations were found to be 35.69% and 32.83%, respectively, in 0.1% SLS solution whereas in the pH 6.8 phosphate buffer, the cumulative drug release was 31.55% and 28.72%, respectively (Figure 4). On comparing the dissolution profile of the drug in solid dispersion form and capsule loaded form in 0.1% SLS medium, it was perceived that marginal improvements in the release have been noticed. Although, the cumulative drug release in pH 6.8 phosphate buffer was found to reduce significantly. The exact reason cannot be explained, however, media features, disintegration characteristics, and polymeric concentrations are believed to play a critical role affecting it.
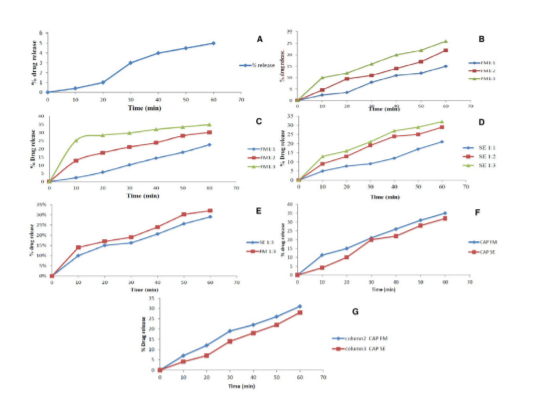
Figure 4. Cumulative drug release characteristics: (A) Fenofibrate pure drug; (B) Physical mixture (Drug + Polymer); (C) Solid dispersion formulation produced by fusion method in 0.1% SLS media; (D) Solid dispersion formulation produced by solvent evaporation method in 0.1% SLS media; (E) Optimized solid dispersion formulations FM 1:3 and SM 1:3 in pH 6.8 phosphate media buffer; (F) Optimized solid dispersion formulations FM 1:3 and SM 1:3 from capsule in 0.1% SLS buffer media; and (G) Optimized solid dispersion formulations FM 1:3 and SM 1:3 from capsule in pH 6.8 phosphate media buffer media.
CONCLUSION
The current research emphasized simple conventional methods (fusion method/melting method and solvent evaporation method) for the formation of solid dispersions of well-known hydrophobic drug fenofibrate us
References:
[1] Mahapatra DK, Bharti SK. Handbook of Research on Medicinal Chemistry: Innovations and Methodologies. New Jersey: Apple Academic Press, 2017.
[2] Mahapatra DK, Bharti SK. Medicinal Chemistry with Pharmaceutical Product Development. New Jersey: Apple Academic Press, 2019.
[3] Borkar SS, Mahapatra DK, Wakodkar SB, Baheti JR. Pharmacology-III. Nagpur: ABD Publications Private Limited, 2020.
[4] Chhajed SS, Bastikar V, Bastikar AV, Mahapatra DK. Computer-Aided Drug Design. Pune: Everest Publishing House, 2019.
[5] Chhajed SS, Upasani CD, Wadher SJ, Mahapatra DK. Medicinal Chemistry. Nashik: Career Publications Private Limited, 2017.
[6] Mahapatra DK, Bharti SK. Drug Design. New Delhi: Tara Publications Private Limited, 2016.
[7] Shivhare RS, Mahapatra DK. Medicinal Chemistry-II. Nagpur: ABD Publications Private Limited, 2019.
[8] Puranik MP, Mahapatra DK. Medicinal Chemistry-III. Nagpur: ABD Publications Private Limited, 2020.
[9] Karen HD, Prajapti PH, Chaudhary JI. BCS Classification and Solubility Enhancement Techniques for BCS Class II and BCS Class IV drugs. Eur J Biomed 2019;6(1):663-670.
[10] Bhaskar R, Monika OL, Ghongade RM. Solid Dispersion Technique for Enhancement of Solubility of Poorly Soluble Drug. Indian J Pharm Biol Res 2018;6(2):43-52.
[11] Singh G, Kaur L, Gupta GD, Sharma S. Enhancement of the Solubility of Poorly Water-Soluble Drugs through Solid Dispersion: A Comprehensive Review. Indian J Pharm Sci 2017;79(5):674-687.
[12] Das PS, Verma S, Saha P. Fast dissolving tablet using solid dispersion technique: a review. Int J Curr Pharm Res 2017;9(6):1-4.
[13] Dhawale P, Mahajan NM, Mahapatra DK, Mahajan UN, Gangane PS. HPMC K15M and Carbopol 940 mediated fabrication of ondansetron hydrochloride intranasal mucoadhesive microspheres. J Appl Pharm Sci 2018;8(8):75-83.
[14] Dangre PV, Godbole MD, Ingle PV, Mahapatra DK. Improved Dissolution and Bioavailability of Eprosartan Mesylate Formulated as Solid Dispersions using Conventional Methods. Indian J Pharm Edu Res 2016;50(3): S209-S217.
[15] Kumar B. Solid Dispersion-A Review. PharmaTutor. 2017;5(2):24-29.
[16] Sharma DK. Solubility enhancement strategies for poorly water-soluble drugs in solid dispersions: A review. Asian J Pharm 2016;1(1):9-19.
[17] Khan S, Gangane PS, Mahapatra DK, Mahajan NM. Natural and Synthetic Polymers assisted Development of Lurasidone Hydrochloride Intranasal Mucoadhesive Microspheres. Indian J Pharm Edu Res 2020;54(1):213-222.
[18] Mahajan NM, Zode GH, Mahapatra DK, Thakre S, Dumore NG, Gangane PS. Formulation development and evaluation of transdermal patch of piroxicam for treating dysmenorrhoea. J Appl Pharm Sci. 2018;8(11):35-41.
[19] Mahajan NM, Wadhwane P, Mahapatra DK. Rational designing of sustained-release matrix formulation of Etodolac employing Hypromellose, Carbomer, Eudragit and Povidone. Int J Pharm Pharm Sci 2017;9(12):92-97.
[20] Gangane PS, Ghughuskar SH, Mahapatra DK, Mahajan NM. Evaluating the role of Celosia argentea powder and fenugreek seed mucilage as natural super-disintegrating agents in gliclazide fast disintegrating tablets. Int J Curr Res Rev 2020;12(17):101-108.
[21] Kazi FS, Mahajan RK, Mahapatra DK, Mahajan UN. Formulation development of innovator equivalent extended release tablets of gliclazide: A way ahead to Generic medicines. J Pharm Sci Pharmacol 2017;3:1-8.
[22] Patil MD, Mahapatra DK, Dangre PV. Formulation and in-vitro evaluation of once-daily sustained release matrix tablet of nifedipine using rate retardant polymers. Inventi Impact Pharm Tech 2016;4:190-196.
[23] Mahajan NM, Pardeshi A, Mahapatra DK, Darode A, Dumore NG. Hypromellose and Carbomer induce bioadhesion of Acyclovir tablet to vaginal mucosa. Indo Am J Pharm Res 2017;7(12):1108-1118.
[24] Godbole MD, Mahapatra DK, Khode PD. Fabrication and Characterization of Edible Jelly Formulation of Stevioside: A Nutraceutical or OTC Aid for the Diabetic Patients. Inventi Rapid: Nutraceut. 2017;2017(2):1-9.
[25] Umaredkar A, Dangre PV, Mahapatra DK, Dhabarde DM. Fabrication of chitosan-alginate polyelectrolyte complexed hydrogel for controlled release of cilnidipine: A statistical design approach. Mater Technol 2018;1:1-11.
[26] Pusala SV, Gangane PS, Mahapatra DK, Mahajan NM. Hydrophilic and Hydrophobic Matrix System Engineered Development of Extended Release Tablets of Oxybutynin Chloride. Int J Pharm Sci Res 2020;11(9):4603-4611.
[27] Gangane PS, Kadam MM, Mahapatra DK, Mahajan NM, Mahajan UN. Design and formulating gliclazide solid dispersion immediate release layer and metformin sustained release layer in bilayer tablet for the effective postprandial management of diabetes mellitus. Int J Pharm Sci Res 2018;9(9):3743-3756.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License