IJCRR - 8(1), January, 2016
Pages: 51-54
Print Article
Download XML Download PDF
COMPARISION OF THE ADVERSE EFFECTS PROFILE OF TRAVOPROST VS BRIMONIDINE/TIMOLOL IN PATIENTS OF PRIMARY OPEN ANGLE GLAUCOMA IN CENTRAL IDIAN POPULATION OF BHOPAL
Author: Rekha Mehani, Major V. K. Yadav, Rajnish Kumar Sankadia, Sarang Ghodki, Tanu Garg
Category: Healthcare
Abstract:Aim: To compare the adverse effects profile of Travoprost vs Brimonidine/Timolol in patients of Primary Open Angle Glaucoma(POAG) Methodology: In this randomized open label 12-week study, 70 patients were randomized to receive either 0.004% of Travoprost once daily in the evening or Brimonidine/Timolol combination twice daily. Their safety was concluded by monitoring their adverse effects during follow-up visits at 2, 4, 8, and 12 weeks. Results: Both treatment regimens were well tolerated during the period of study. Patients in the Travoprost group had a higher incidence of ocular irritation (30%), pigmentation of skin (33%), Thickening of eyelashes (39%) and hyperemia (36%) when compared to the Brimonidine/Timolol group which showed only ocular irritation (21%) and foreign body sensations (28%). Conclusions: The incidence of adverse effects were not significantly different between Travoprost and Brimonidine/Timolol therapy. Both had favourable safety profiles. However, Travoprost had more local side effects profile when compared to Brimonidine/Timolol.
Keywords: Beta blocker, Intraocular pressure, Prostaglandin analogue, ?2 agonist, Adverse effects
Full Text:
INTRODUCTION
Glaucoma is an optic neuropathy characterized by acquired loss of retinal ganglion cells and atrophy of the optic nerve leading to vision loss. Glaucoma is the second leading cause of blindness globally, (Quigley HA. 1996) accounts for 12.3% of total blindness (Congdon N et al). Elevated intraocular pressure (IOP) is a primary risk factor both for the development of glaucoma and for progression of optic nerve changes and visual field loss in the disease (Morrison JC. 2005). Primary open-angle glaucoma (POAG), the most common type of glaucoma is characterized by chronically elevated IOP with no known cause for the elevated IOP or optic neuropathy.
The aim of treatment in glaucoma is to reduce IOP. Recent randomized, controlled clinical trials have shown that lowering IOP is effective in delaying or preventing the development of glaucoma in patients with ocular hypertension (OHT) and in delaying or halting the progression of established glaucoma.(Haijl et al 2002, Kass MA et al 2002). Further IOP reduction is beneficial in reducing the risk of progression of vision loss even when IOP is already within the normal range (Collaborative Normal-Tension Glaucoma Study Group 1998). Evidence suggests that very low IOP provides the best visual outcomes for patients.
(The AGIS Investigators 2000; Lichter et al 2001). Analysis of data from the Early Manifest Glaucoma Trial showed a 10% reduction in the risk of progression associated with each 1 mmHg of IOP reduction (Leske et al 2003). Medical treatment is the first line of management and includes the use of several classes of topical agents. Brimonidine /Timolol is the commonly used drug combination to reduce IOP. Travoprost, a prostaglandin analogue has come up with powerful ocular hypotensive effects (DuBiner HB, Laurence L Bruton)9,10 The objective of present study was to compare the safety profile of Travoprost with Brimonidine / Timolol in patients with POAG.
METHOD All the cases studied were attending the outpatient Department of Ophthalmology at Peoples Medical College Bhanpur Bhopal. Study duration was 18 months. While selecting the cases for the study special care was taken to include only newly diagnosed cases of POAG. Necessary approval were taken (CTRI/2011/11/002105). Informed consent from the patient was obtained after explaining to them the details of the study. Inclusion criteria are patients with Primary open angle glaucoma aged more than 40years with IOP more than 21mmHg having visual acuity 6/24 or better.
Patients with inflammation, ocular infection, advanced cataract and patients of bronchial asthma and cardiac disease were not included in the study. Patients were divided into two groups Group A and B based on simple random sampling. One group was treated with 0.004% of Travoprost eye drops once a day in the morning and the other with fixed combination of Brimonidine/Timolol eye drops twice a day. The intraocular pressure was measured at baseline, 2, 4, 8, and 12 weeks of the visit using Applanation tonometer and side effects were recorded. Total 5 patients did not come for the follow up and study was completed in 65 patients (Group A n = 33,Group B n = 32 ).
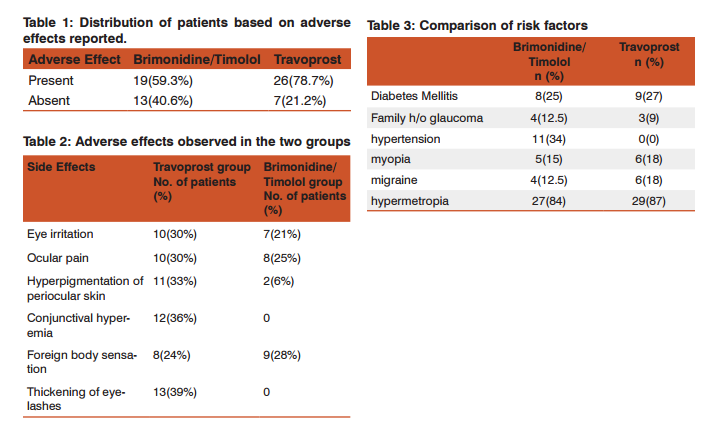
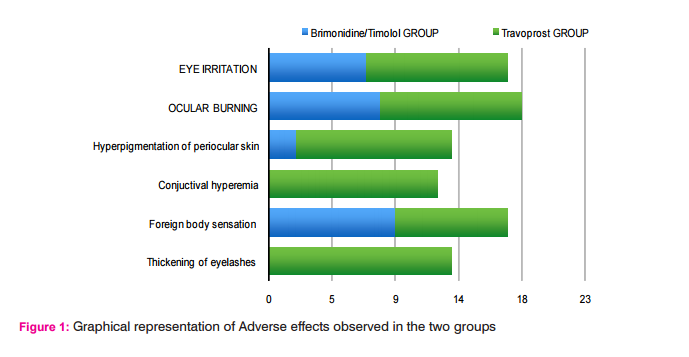
RESULTS Both treatment regimens were well tolerated during the study. Patients in the Travoprost group had a higher incidence of ocular irritation (30%), pigmentation of skin (33%), Thickening of eyelashes (39%) and hyperemia (36%) when compared to the Brimonidine/Timolol group which showed only ocular irritation (21%) and foreign body sensations (28%). There were no serious side- effects observed in this study. Both the drugs were well tolerated.
Travoprost had a higher incidence of adverse effects. ocular irritation (30%), pigmentation of eyelashes (36%), Change of iris colour (25%), Thickening of eyelashes (39%)and hyperpigmentation (33%) when compared to the Brimonidine/Timolol group which showed only ocular irritation (21%). There was no ocular pain with Brimonidine/Timolol, in contrast to the Travoprost group where 10% of the patients suf fered from ocular pain. Conjunctival hyperemia (36%) with Travoprost. From our observations Travoprost appears to have more adverse effects.
DISCUSSION Travoprost acid, the biologically active form of Travoprost, is a prostaglandin F2α analog and a fully selective agonist to the prostaglandin F receptor (Laurence L Bruton Franks WA, Sharif NA 2003, Sharif NA 2003, Hellberg MR et al). It reduces IOP by increasing the outflow of aqueous humor through the uveoscleral pathway (Schachtschabel U et al 2000, Denis P et al 2007). In studies to evaluate the relative incidence of hyperemia between prostaglandins, Netland et al found that the incidence of hyperemia caused by latanoprost was 27.6%, while Travoprost had a rate of 49.5%, and Cantor et al observed hyperemia in 21.1% of eyes treated with bimatoprost and in 14.8% in eyes treated with Travoprost (Netland et al, Cantor LB et al 2006).The use of Travoprost 0.004% induced a conjunctival hyperemia incidence of 32.5%–49.5%, whereas timolol maleate 0.5% treatment had an incidence of 7%–14%(Denis P et al 2007 , Goldberg I et al 2001).In our study we found hyperemia in 36% of patients which is comparable to other studies. Hyperemia was usually mild and unlikely to interrupt clinical studies. In fact, improvement of hyperemia during continued dosing has been reported (Goldberg I et al 2001).
Prostaglandin analogues (PGA) can also induce darkening of the iris, and its incidence in the literature varies according to the different methods used in its evaluation. Netland et al also reported that iris hyperpigmentation was observed in 5.2% of eyes treated with latanoprost and in 3.1% in eyes treated with Travoprost and in our study it was more that is 33%. It is much more higher as compared to other studies. The eyelashes can suffer some changes during treatment with Travoprost and other PGAs. These drugs can induce eyelashes to increase in number and can cause alteration in their length, thickness, and darkness. In our study we found thickening of eyelashes in 39% of patients. Other body systems are not considerably affected by Travoprost treatment (Denis P et al 2007 ).
Brimonidine is a selective α-2 adrenergic receptor agonist. Brimonidine has a dual mechanism of IOP lowering: it both reduces aqueous humor production and stimulates aqueous humor outflow through the uveoscleral pathway (Toris CB et al). Brimonidine has a favourable safety and tolerability profile. Unlike the β-adrenergic antagonists, there are no cardiopul- monary contraindications to its use, and Brimonidine can be safely and effectively used by patients on systemic antihypertensive beta-blocker therapy (Schuman JS. 2000). In contrast to the prostaglandin analogues, Brimonidine has not been associated with eyelash growth or increased pigmentation of the iris or eyelids.
The side- effects associated with Brimonidine treatment are usually ocular burning, foreign body sensation, allergic conjunctivitis, and ocular pruritus. The most common systemic side-effects are oral dryness and fatigue or drowsiness. Brimonidine/Timolol fixed combination also proved better in terms of number of adverse events as observed in both groups, with 20% more adverse events seen in the Travoprost group (Table 1). Brimonidine/ Timolol fixed combination has more foreign body sensation than Travoprost group. There were no serious, unexpected, related adverse events reported for any therapy.
Timolol reduces intraocular pressure (IOP) by suppression of aqueous secretion and prevents damage to optic nerve and subsequent loss of vision.10 Side effects are stinging, redness and dryness of eye, corneal hypoesthesia, blurred vision and allergic blephero-conjunctivitis. Ocular pain was almost similar in both groups. From our observations Travoprost appears to have more adverse effects.
CONCLUSION Travoprost and Brimonidine/Timolol are proven drugs for the treatment of Primary open angle glaucoma. The choice of drug will depend on many factors including the adverse effect profile. Present study demonstrated a favourable safety profile for the Brimonidine/Timolol combination which produced fewer local adverse effects. however Brimonidine/ Timolol combination has to be avoided in the patients of bronchial asthma for the fear of precipitation of asthma attack.
ACKNOWLEDGEMENT Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
1. Cantor LB, Hoop J, Morgan L, et al. Intraocular pressure-lowering efficacy of bimatoprost 0.03% and Travoprost 0.004% in patients with glaucoma or ocular hypertension. Br J Ophthalmol. 2006;90(11):1370–1373.
2. Congdon N, O Colmain B, Klaver CC, et al. causes and prevalence of visual impairment among adults in united states. Arch Ophthalmol, 2004, 122:477-85
3. DuBiner HB, Sircy MD, Landry T, et al. Comparison of the diurnal ocular hypotensive efficacy of Travoprost and latanoprost over a 44-hour period in patients with elevated intraocular pressure. ClinTher 2004; 26:35-47.
4. Denis P, Covert D, Realini A. Travoprost in the management of open-angle glaucoma and ocular hypertension. Clin Ophthalmol. 2007;1(1)11–24.
5. Franks WA, Renard JP, Cunliffe IA, et al. A 6-week, doublemasked, parallel-group study of the efficacy and safety of Travoprost 0.004% compared with latanoprost 0:005%/timolol 0.5% in patients with primary open-angle glaucoma or ocular hypertension. Clin Ther. 2006;28(3):332–339.
6. Goldberg I, Cunha-Vaz J, Jakobsen JE, et al. Comparison of topical Travoprost eye drops given once daily and timolol 0.5% given twice daily in patients with open-angle glaucoma or ocular hypertension. J Glaucoma. 2001;10(5):414–422.
7. Heijl A, Leske MC, Bengtsson B, et al. Early Manifest Glaucoma Trial Group. Reduction of IOP and glaucoma progression results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268–1279.
8. Hellberg MR, Sallee VL, McLaughlin MA, et al. Preclinical efficacy of Travoprost, a potent and selective FP prostaglandin receptor agonist. J Ocul Pharmacol Ther. 2001;17(5):421–43
9. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13. [PubMed]
10. Laurence L. Bruton editor. Goodman and Gilman`s The Pharmacological Basis of Therapeutics, 12th edition, ocular pharmacology, New York Mc Graw Hill 2011.
11. Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48–56. [PubMed]
12. Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–53. [PubMed]
13. Morrison JC. 2005. Elevated intraocular pressure and optic nerve injury models in the rat. J Glaucoma, 14:315-7.
14. Netland PA, Landry T, Sullivan EK, et al. Travoprost compared with latanoprost and timolol in patients with open-angle glaucoma or ocular hypertension. Am J Ophthalmol. 2001;132(4):472– 484.
15. SchachtschabelU, LindseyJD, WeinrebRN. Themechanismofaction of prostaglandins on uveoscleral outflow. Curr Opin Ophthalmol. 2000;11(2):112–115.
16. Schuman JS. 2000. Effects of systemic beta-blocker therapy on the efficacy and safety of topical brimonidine and timolol. Ophthalmology, 107:1171-7. Quigley HA. 1996. Number of people with glaucoma worldwide.Br J Ophthalmol, 80:389-93.
17. Sharif NA, Crider JY, Husain S, et al. Human ciliary muscle cell responses to FP-class prostaglandin analogs: Phosphoinositide hydro- lysis, intracellular Ca2+ mobilization and MAP kinase activation. J Ocul Pharmacol Ther. 2003;19(5):437–455.
18. Sharif NA, Kelly CR, Crider JY, et al. Ocular hypotensive FP prostaglandin (PG) analogs: PG receptor subtype binding affinities and selectivities, and agonist potencies at FP and other PG receptors in cultured cells. J Ocul Pharmacol Ther. 2003;19(6):501–515.
19. The AGIS Investigators. The advanced glaucoma intervention study (AGIS) 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130(4):429–440.
20. Toris CB, Gleason ML, Camras CB, et al. Effects of brimonidine on aqueous humor dynamics in human eyes. Arch Ophthalmol. 1995;113:1514–7. [PubMed].
21. Quigley HA. 1996. Number of people with glaucoma worldwide.Br J Ophthalmol, 80:389-93.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License