IJCRR - 8(1), January, 2016
Pages: 46-50
Print Article
Download XML Download PDF
SYNTHESIS AND CHARACTERIZATION OF DIPHENYLTIN (IV) COMPLEXES WITH CYCLOPROPANE AND CYCLOPENTANE CARBOXYLIC ACIDS
Author: Tanzeel Ur Rehman, Misbah Zahid
Category: Healthcare
Abstract:Aim: Compounds of diphenyltin carboxylates were prepared by using two different ligands i.e. cyclopropane and cyclopentane. Materials and Method: Solvent used for this purpose in CDCl3. The NMR spectra of 13C and 119Sn of CDCl3 solution of these compounds have been recorded. FTIR spectra are also recorded. CHNS analysis has been done to record the percentage of carbon and hydrogen in these compounds. Results: NMR and FTIR spectra are recorded and percentage of carbon and hydrogen was obtained by using elemental analysis. Conclusions: The values of the chemical shifts of the diphenyltin group give the proof that these compounds are monomeric species within the solutions no matter of their structure within the solid state.
Keywords: Diphenyltin carboxylates, Cyclopropane, Cyclopentane, FTIR spectra
Full Text:
INTRODUCTION
Organometallic compounds contain at least one C-Metal bond. Organometallic compounds are also known as metalorganics, metallo-organics and organo-inorganics. All Gillman and Grignard reagents are also examples of such organometallic compounds. Organometallic compounds contain also transition metals. Ferrocene and Tetracarbonyl nickel are examples of such organometallic compounds. Boron, silicon, arsenic, and selenium are also considered to form organometallic compounds along with other traditional metals and semimetals [1].
Organometallic compounds of Tin
Compounds containing tin with hydrocarbon substituents are called organotin compounds or stannanes. Organotin chemistry consists of wider field of organometallic chemistry. Diethyltindiiodide was the first organotin compound, discovered by Edward Frankland in 1849 [2]. Organotin compounds are classified as R4 Sn, R3 SnX, R2 SnX2 and RSnX3 .
In compounds of industrial importance, R is usually a butyl, octyl-, or phenyl group and X, a chloride, fluoride, oxide, hydroxide, carboxylate, or thiloate. Organotin compounds have one or more carbon-tin covalent bonds that are responsible for the specific properties of these molecules. Essentially all organometallic tin compounds are of the Sn (IV) type. The only well established compound with tin having the oxidation state +2 is the tin (II) cyclopentadienyl (C10H10Sn). There are four classes of organotin compounds depending on the number of alkyl group. These series are designated as mono-, di-, tri-, and tetraorganotin compounds [3].
Uses and applications of organotin compounds
1. Organotin compounds can be powerful bactericides and fungicides depending on the organic groups. [4] 2. Tributyltins are utilized as industrial biocides, for example, as antifungal agents in breweries, industrial cooling systems. These are used in textiles and paper, paper mill systems and wood pulp. These are also used in marine anti-fouling paint [5, 6].
3. Organotin compounds are widely studied class of meta-based antitumor drugs [7].
4. Triphenyltins are applied as active components of agricultural fungicides and antifungal paints [8].
5. Organotin compounds are used in the treatment of hyperbilirubinaemia [9].
6. Organotin compounds are used for wood preservation [10].
7. Addition of Organotin compounds to PVC increases its stability [11, 12].
8. Organo metallic transition complexes also have a role in molecular rearrangement processes [13].
Organotin carboxylates
As for organotin carboxylates are concerned, Sn exists in two oxidation states Sn(II) and Sn(IV) in these compounds. Sn(II) formate and acetate are made by simple methods. Tin acetate in the oxidation state of two is stable and can be stored for some months, but over longer periods of time it undergoes slow oxidation. It hydrolyses slowly over several hours in water, but the adduct (CH3 COO)2 Sn. CH3 COOH is formed by dissolving Sn(II) acetate in glacial acetic acid. This adduct is unhydrolysed, even in boiling water. In addition to these simple ones, evidence has been presented for many more carboxylate species of Sn((II). Thus CH3 COOSn, (CH3 COO)2 Sn, (CH3 COO)7 Sn, (CH3 COO)5 Sn and (CH3 COO)3 Sn have been reported. The tri-acetatostanates have been well characterized. The organotin (IV) carboxylates are of particular interest in a way that they appear to exist in more than one form [14].
For example when triphenyltin chloride reacts with, for example, formic acid and acetic acids, the products [(Ph)3 SnOOCH and (Ph)3 SnOOCH3 ] are very insoluble. These insoluble products are believed to be associated by bridging carboxylate groups, either as ring or linear polymers [15]. If however, the polymeric forms of these are heated in cyclohexane at 100° C for several hours, they are both converted to new soluble forms. A remarkable point of interest is that the soluble and insoluble forms of these organotin carboxylates are sublimable at high vacuum with out Interco version [16]. IR is one of the most important spectroscopic methods used for qualitative and quantitative analysis.
It is based on the fact that each compound has its own unique spectra and certain functional groups absorbent about the same wavelength even in different molecules. Its single most important use has been made for the identification of organic compounds whose spectra are generally complex and provide numerous maxima and minima that are used for comparison purposes. Indeed in most instances, the IR spectrum of the compounds especially of organic compounds provides a unique finger print, which is readily distinguished from the absorption pattern from all other compounds because only optical isomers absorb in the same way.
Absorption of IR radiation is confined largely to molecular species for which small energy differences exist between various vibration and rotational states. NMR is an important technique used for the analysis of compounds. Like electron, nucleons also have spin .If an atom has an even number of nucleons, the spins cancel out and there is no overall magnetic moment [17]. If however, an atom has an odd number of nucleons, there is an overall magnetic moment. As a result, the nucleus can take up one of the two orientations whose energy splits in the presence of an external magnetic field, this splitting of the energy form the basis of nuclear magnetic resonance and NMR spectroscopy. Elemental analysis is an experimental work to determine the amount of an element in a compound in weight percent.
Different experiments are used to determine elemental composition as there are many different elements. CHN (carbon, hydrogen, and nitrogen) is the most common type of elemental analysis. CHN analysis is especially useful for organic compounds. The elemental analysis of a compound is used for the determination of the empirical formula of the compound. The formula which shows the simplest whole number ratio of the elements in a compound is called empirical formula. Each CHN analysis should include at least 5 mg of transferable sample. CHN analysis provides a quick and inexpensive method to check sample purity, and in conjunction with mass spectrometry and NMR data, can be used to characterize a compound.
EXPERIMENTAL
Chemicals used Dry Toluene, Diphenyltin (IV) oxide were purchased from Riedal-de Hean and used with out further purification. Instrumentation IR (FTIR, 3000MX, Bio-Rad Excalibur Series, USA), 1 H AND 13C NMR (Advance 300 MHZ NMR, Bruker). Procedure A (0.5 ml, 2mmol) of cyclopropane carboxylic acid was dissolved in dry toluene in round bottom flask two-necked flask. To this solution, Ph2 SnO (0.91, 1mmol) added with constant stirring. The solution was refluxed for four hours. Water produced as a by product was removed through Dean Stack apparatus. Solvent was evaporated by rotary evaporator under reduced pressure. Solid obtained was dried in air.

ELEMENTAL ANALYSIS As for as compound 1 is concerned, the calculated amounts of carbon and hydrogen are 54.21% and 4.52% respectively and found amounts are 46.56% and 4.63% respectively. In case of compound 2, the calculated amounts of carbon and hydrogen are 54.21% and 4.52% respectively and found amounts are 46.56% and 4.63% respectively.
DISCUSSION
IR spectroscopy
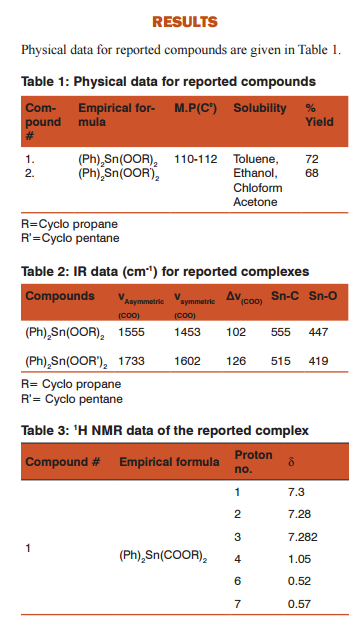
As for as spectrum is concerned we see a prominent peak at 450cm-1in Table-2, which indicates the formation of metal to oxygen bond. Another change that we observe in this spectra as compared to ligand spectra is stretching vibration of C=O, which normally occurs at 1700cm-1 but these are shifted to lower wavelength side because when metal oxygen bond forms, most of the electron density is shifted to metal and as a result the bond is strengthen and vibration occurs at lower wavelength side. This bond formation is also evidenced by the fact, in this spectrum, the peak also appears as consequences of the M-O bond formation. Another important thing that we determine from this spectrum is the coordination number of the central atom. For this we calculated the value of the V which is determined by measuring the difference between the symmetric and asymmetric vibrations.
As shown in the above table, peaks are observed at 555 cm-1, 515 cm-1 and 477 cm-1, 419 cm-1 for Sn-C and Sn-O bonds for compounds 1 and 2 respectively. Another thing observed the value for ?v which to be 102 cm-1 and 126 cm-1, these values indicate that the ligands act as a monodentate ligand. Similarly another peak which appears at 3300-2500 cm-1for O-H is disappeared in case of the spectrum of the complex due to the dissociation of this proton. The formation Sn-O bond is also evident by the fact that a peak is observed for C-O. This peak is appeared because after bond formation most of the electron density is shifted to Sn as a result C=O changes to C-O.

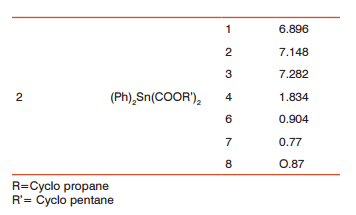
It is evidence from the table-3, in the first complex peak for proton 1, 2, 3, 4 and 5 are at 6.896 ppm, 7.148 ppm, 7.282 ppm, 1.834 ppm and 0.904 ppm. The spectrum is complex due to carboxylato and alkyl groups attached to the metal which are very bulky. In the case of second compound it is evidence from the table that in the first complex, peaks for proton 1, 2, 3, 4 and 5 are at 6.896 ppm, 7.148 ppm, 7.282 ppm, 1.834 ppm and 0.904 ppm.
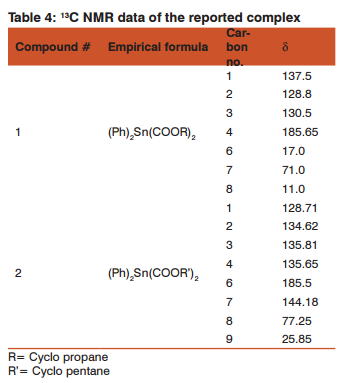
It is evidence from the table-4 that in the first complex peaks for carbon 1, 2, 3, 4, 6, 7 and 8 are at 137.5 ppm, 128.8 ppm, 130.5 ppm, 185.65 ppm, 17.0 ppm, 71.0 ppm, and 11.0 ppm respectively. For the second complex, peaks for carbon 1, 2, 3, 4, 6, 7, 8 and 9 are at 128.71 ppm, 134.62 ppm, 135.81 ppm, 135.65 ppm, 185.5 ppm, 144.18 ppm, 77.25 ppm and 25.85 ppm respectively. The spectrum is complex due to carboxylato and alkyl groups attached to the metal are very bulky. As for as very bulky groups are attached to metal, so from 13C NMR and proton NMR spectra we could not easily identified the metal to ligand bond and complex formation.
Elemental Analysis The difference in amounts for compound 1 may be due to impurities or instrumental error. For compound 2, amounts are almost equal.
CONCLUSION These compounds are monomeric species within the solutions no matter of their structure within the solid state.
ACKNOWLEDGEMENT Authors are thankful to Professor Dr. Saqib Ali, Head of Department Quaid-I-Azam University, Islamabad, Pakistan whose advice made energized to do this work. Authors acknowledge the huge help received from the technical staff of the QAU laboratory for NMR analyses. Authors accede the immense advice accustomed from the scholar community whose online writing are cited and included in references of this manuscript. The authors appreciate authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
1. P. Powell, Principles of Organometallic Chemistry, (1988), 2nd edition, Chapman and Hall, New York, pp-1-10.
2. Synthetic aspects of tetraorganotins and organotin (IV) halides Sander H.L. Thoonen, Berth-Han Deelman, Gerard van Koten Journal of Organometallic Chemistry 689, 2145-2157 (2004).
3. T. Mole and E.A Jaffery, Organometallic Compounds, (1972), 3rd edition, Elsvier Publishing Company, London, pp.89-92.
4. J.C Bailar, H.J Emeleus, S.R Nhylom and A.F Trotman, Comprehensive Inorganic Chemistry, (1973), 2nd edition, Prgmon, New York, pp.153-157.
5. Investigations on organo-tin compounds III. The biocidal properties of organo-tin compounds, G.J.M. van der Kerk, J.G.A. Luitjen, J. Appl. Chem. 4 (1954) 314-319.
6. O. S. AYANDA, O. S. FATOKI , FOLAHAN, A. ADEKOLA and BHEKUMUSA J. XIMBA Chem. Sci. Trans., 2012, 1(3), 470-481.
7. F.Arjmand, S. Parveen, S Tabassum,C. Pettinari, Inorganica Chimica Acta 423 (2014) 26–37.
8. J.C Bailar, H.J Emeleus, S.R Nhylom and A.F Trotman, Comprehensive Inorganic Chemistry, (1973), 4th edition, Prgmon, New York, pp.89-93.
9. T. Mole and E.A Jaffery, Organometallic Compounds, (1972), 3rd edition, Elsvier Publishing Company, London, pp.89-92.
10. Davies, Alwyn George. (2004) Organotin Chemistry, 2nd Edition Weinheim: Wiley-VCH. ISBN 978-3-527-31023-4
11. E. Ark?xs, D. Balkose, Polymer Degradation and Stability, 88, (2005), pp. 46-51.
12. G. Ayrey, B.C. Head, R.C. Poller, Macromol. Rev. 8 (1974) 1.
13. J.F. Harrod, R.M. Laine ,Applications of Organometallic Chemistry in the Preparation and Processing of advanced materials, Cap D’Agde France, 1994, pp. 194-195.
14. M. Mazhar, M.A. Choudhary, S. Ali, X. Qing-Lan and S. Xincging, J. Chem. Society of Pakistan. 23, 103-131 (2001).
15]. M. Gielen, Coordination Chemistry. Rev. 151, 41-51 (1996).
16. J.W Robinson, Undergraduate Instrumental Analysis, 5th Edition, (995). 17. A.U Rehman, Nuclear Magnetic Resonance, 1st edition, (1989), pp.90-93.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License