IJCRR - 8(1), January, 2016
Pages: 36-45
Print Article
Download XML Download PDF
STUDY ON THE IMPACT OF DRUGS ON HEART AND CARDIO-VASCULAR SYSTEM IN CRABS
INHABITING FRESHWATER, MARINE AND BRACKISH WATERS
Author: Premraj Loganathan, M. Ranjani Devi and Dr. J.M.V. Kalaiarasi
Category: Healthcare
Abstract:Aim: The present study was aimed to evaluate the effects of various drugs on different in habitat crabs Methods: The sterile syringes was loaded with different chemicals and poured over the heart of the dissected animal. The stop watch was switched on again and the number of beats per minute was noted. The rate of heart beats was plotted in relation to time exposure in four chemicals like Adrenaline, Atropine, Ephedrine and Ringer solution. The plotted graphical interpretation has been given the nature of chemical and their intensity and finally removed the hearts from the treated animal were subjected to histopathology and haemolymph preparations. Results: The hearts of the three different crustaceans were isolated for the histopathology process to study, diagnose and to differentiate between the cardiac muscles of the crabs. It was observed that edema was seen in all the smears but it was higher in marine species than the estuarine and freshwater species. Inflammation was also seen but except marine species, the others show inflammation. There were no toxic accumulation and pathogens observed and haemolymphs was observed that the smears were even and contains many cells which are irregular in their shape and size. Conclusion: From the results of study we conclude that, Adrenaline, Atropine, Ephedrine and Ringer solutions initially inhibit the heart rate which indicates animal try to settle-down into the chemical medium but later on the effects of toxicants accelerate the metabolic activity of the animals which accelerate the rate of heart beat in a crab after this treatment. Then the heart rate slowly decreases as the animal dies.
Keywords: Carcinusmaenas, Scylla serrata, Parathelphusapantherina, Histopathology and haemoloymph
Full Text:
INTRODUCTION
Crustaceans possess an open type of circulatory system and the haemolymph flows in the blood sinuses. A dorsally situated heart is present in most of the crustaceans. Data available on the rate of heart beat in crustaceans suggest that the heart rate is influenced by a number of factors like body size , activity, respiration, stress, light, blood composition, temperature , nutritional status, population density, amount and the kind of food, oxygen and carbon dioxide content of medium, moulting, diurnal cycle, PH, internal pressure etc. By far the best studied factor that influences heart beat rate is body size. As a general rule, it might be stated that the heart rate varies inversely with body size. Thus the rate of heart beat is faster in smaller animals of a species and slower in larger species.
The Q10 of the rate of heart beat is affected by sex is shown long time before and it might also mean that the rate of heart beat is different in the two sexes. Effects of different heavy metals on biochemical constituents in fishes and other aquatic animals is the current topic of interest, probably because of the contamination of natural water resources by these pollutants from various industrial effluent disposals, threatening the fish culture and population. Action of pesticides, heavy metals is stress on the non-target organisms which induce the changes in them. Any changes to come over the stress needs energy, normally various sources of energy metabolism are acquainted by the organisms to encounter the stress. The haemolymph or blood is an important tool of transport of these constituents therefore the study of haematological changes in the organism under stress induced by various pollutants assumes importance.
The paucity of information has become particularly apparent in recent years as investigators have become interested in the cardiovascular system of crab with considerable success. The heart rate of marine, estuarine and fresh water crabs during aquatic and aerial respiration was studied. Recently valuable contribution has been studied the effect of three chemicals, viz. Adrenaline, Atropine and Ephedrine on the heart rate of these crabs. Studied effect of Adrenaline, Atropine and Ephedrine on the isolated hearts of Carcinusmaenas, Scylla serrata and Parathelphusapantherina. In recent year investigators divert their concentration to observe effect of toxicant such as insecticide, heavy metals, drugs and antibiotics upon such sensitive physiological system of different animals. The present study was undertaken to determine the effect of three drugs such as Adrenaline, Atropine and Ephedrine on the heart rate of freshwater, estuarine and marine crabs.(Arunaet al., 2011). Crustaceans possessing many primitive features tend to have myogenic hearts, although neurogenicity is dominant in the more advanced malacostracan groups (Wilkens, 1999a; Yamagishi et al., 2000) and possibly members of the Ostracoda. Exactly how the heart is regulated, via neuronal and neuro-hormonal controllers, has recently been investigated and there is a long history of examining the effect of environmental factors (e.g., hypoxia, salinity) on aspects of cardiac function (McMahon, 1999a, 2001; De Pirroet al., 1999).
The animals required food and oxygen continuously for energy and to perform various metabolic activities. Thus digested food and oxygen should be transported to all the cells. This function is carried out with the help of body fluids. The arthropods possess the open type of circulatory system, which is presumably derived from the highly organized closed system of their annelids or pre-annelids ancestors. In most of the crustaceans the heart is dorsally placed inside the body. Whatever knowledge about cardio physiology of the crab does not fulfill the existing gap. Now-a-days investigators divert their concentrations to observe effects of toxicants pesticides upon the sensitive physiological system of animals. (Deshai R. B, Shinde V. D, Katore B. P, et al., 2012). The following experiments, which include a study of the action of certain drugs and autacoids upon the hearts of crabs, were undertaken as a preliminary to a reinvestigation of the nervous supply to the crustacean heart and its relationship to the heart-beat.
The results obtained are of interest in view of the paucity of our knowledge regarding the effects of such substances on the heart of invertebrates. Since the investigation of the action of adrenaline and ephedrine formed the starting-point of the present study, the literature relating to this subject will first be dealt with, other work being noted and references given as the occasion arises. It would seem that Carlson was the first to record a positive action of adrenaline on the heart of an invertebrate, observing an excitatory effect on both the cardiac ganglion and myocardium of a marine crab, but giving no graphic record of his experiments. Elliot obtained negative results in a single experiment on the crayfish, in which he dropped adrenaline on to the surface of the exposed heart without effect. He does, however, state that “movements of the animal followed as the drug was carried round in the circulation and irritated the nervous ganglia,” this being observed also by Brucke and Satake in an experiment on Homarus, a rise in bloodpressure and increased heart-rate (following an initial fall in blood-pressure after an injection of adrenaline) coinciding with the commencement of these movements.
More recently Hogben and Hobson have studied the action of adrenaline on a wide selection of invertebrate preparations, the Crustacea being represented by the decapods Cancer maenus. In the isolated heart of this animal, perfused with artificial seawater of suitable reaction, these authors recorded an increase in tone of the heart-muscle accompanied by acceleration of the beat in response to adrenaline in a concentration of 1 in 40,000 parts of the perfusing fluid. They state that the latent period between the addition of the autacoid and the onset of the characteristic response was usually somewhat protracted, a minute at least intervening in most experiments, and they consider this evidence that the heart-muscle absorbs the autacoid slowly. Epinine was found by these workers to produce an effect similar to that obtained with adrenaline, but was effective in much greater dilution. (W. A. Bain et al., 1929).A drug may cause paralysis by depressing the motor nerve endings or by paralyzing the central nervous system. My results go to show that the primary action of adrenaline, atropine and ephedrine in arthropods is on the central nervous system and the peripheral ganglia and not on the motor nerve endings in the muscle.
The action on the nerve centers is a primary stimulation followed by temporary or permanent paralysis if the dose is of sufficient strength. The stimulating action of curare on the nerve centers appears immediately on the application of the solution. In the squid the stimulation results in spasms and tetanus. The primary stimulation is less in evidence in the crustaceans. In the gastropods it appears in prolonged and extreme contraction of the body muscles. In all the animals studied the stimulation of the motor nerves causes contraction of the skeletal and visceral muscles after a dose of curare that completely paralyzed the central nervous system. Atropine appears to paralyze motor nerve endings to smooth muscles (example: lungs, gastrointestinal tract) in vertebrates.
The body muscles of arthropods are of the transversely striated type, but the muscles of mollusks approaches more closely to the smooth variety. The experiments on several classes of mollusks with a view of paralyzing the motor nerve endings in the muscle by atropine have yielded uniformly negative results. So far, then, we know of no drugs that will paralyze the motor nerve endings in invertebrates without materially depressing the muscle itself, after previous paralysis of central ganglia. (A. J. Carlson et al., 1922). The neurotransmitter candidates for the intrinsic and extrinsic heart neurons have been identified in a few species for pharmacological and immunecytochemical studies. Dealing with the decapods, Yazawa and Kuwasawa (1994) proposed that gamma- amino - butyric acid (GABA) and dopamine (DA) are the extrinsic neurotransmitters of the cardio-inhibitory and cardio-accelerator nerves, respectively, in the hermit crab Aniculusaniculus. They also proposed that acetylcholine and dopamine are the intrinsic neurotransmitters of the small and large neurons, respectively, in the cardiac ganglion of that species. (Hiroshi Ando, Kiyoaki Kuwasava et al., 2004).
Atropine is the drug of choice for treatment of organophosphate nerve agent and insecticide intoxication and has been used for this indication for several decades. Adverse reactions to atropine may occur, and are of two types: toxic and allergic. Atropine is considered the drug of choice for nerve agent intoxication, since the late 1940s. It continues to be the standard treatment despite the fact that many cholinergic blocking substances have since been tested and found active. Atropine is a competitive inhibitor of the muscarinic acetylcholine receptor. It blocks the effect of excess acetylcholine and protects the receptor from further stimulation. It has a minimal effect at nicotinic receptor sites. Although atropine does not readily cross the blood-brain barrier, the drug has some central beneficial effects in OP poisoning. The central nervous system effects observed in atropine overdose demonstrate that it is capable of crossing the blood-brain barrier to some extent.
Atropine systemic toxicity causes tachycardia, tachypnea, elevated body temperature, and CNS stimulation marked by restlessness, confusion, psychotic reactions, delirium and occasionally seizures. A rash may appear on the face or upper trunk. In severe intoxication, central stimulation may cause CNS depression, coma, circulatory and respiratory failure, and death. (Eyal Robenshtok MD, Shay Luria MD, Zeev Tashma et al., 2002). The first reported use of ephedrine was in China over 5000 years ago as a drug called Ma-huang (meaning astringent yellow). It was mentioned in herbal books as early as 2000 BC. A Chinese medical plant publication in 1596, recommended the drug for improving bad circulation, reducing fever, and treating respiratory ailments. The main ingredient in the Ma-huang was dried green leaves and shoots from Ephedra sinicra.
It is now known that of the 45 species in the Ephedra genus only 25 contain the alkaloid, which can be present in leaves at concentrations of 1-2 %. Today Ephedrine is prepared by synthesis via the reductive amination of Phenyl acetyl carbinol (PAC), which is produced by Saccharomyces cerevisiae during the fermentation of sugar medium containing benzaldehyde. (Aidan J Mullen GRSC MISI et al., 1991).
MATERIALS AND METHODS
SPECIMEN COLLECTION
Young specimens of Carcinusmaenas, Scylla serrataand Parathelphusapantherina were selected for the experiment. The marine and estuarine crabs were bought from Chindadripet market, Chennai and freshwater crabs from Chengalpet in Chennai, Tamil Nadu and were transported to the laboratory in large plastic containers filled with beech water and lake water to minimize stress and mortality. They were acclimatized to standard laboratory conditions for 15 days in de-chlorinated water contained in a large aquarium with proper aeration. Cannibalism was observed during the period of acclimatization.
DIET AND FEEDING
During acclimatization period, crabs were fed with freeze dried tubifex worms in the form of small cubes and also with small pellets once daily. The pellets are composed of the following ingredients (white fish meal, shrimp meal, wheat flour, corn meal, yeast, enzyme, calcium, magnesium, biotin, vitamin A, C, E and other trace elements. Feeding was withheld for 24 hours prior to the commencement of the experiment to keep the experimental animal more or less in the same metabolic state.
REQUIREMENT OF CRABS FOR EXPERIMENT:
After acclimatization, crabs with an average weight of 20 - 50 grams were selected. The crabs were introduced into big plastic tubs which were washed thoroughly. Crabs belonging to both the sexes were used.
CHEMICALS USED:
In the present study, Adrenaline, Atropine, Ephedrine and Ringer solution were used to study its effects on the cardio system of crabs.
RINGER SOLUTION:
The crab ringer prepared had following composition. Sodium chloride: 16.100 gm, Potassium chloride :0.4162gm, Calcium chloride : 0.3403 gm, Magnesium chloride : 0.0804 gm, Sodium sulphate : 1.5261 gm, Sodium Phosphate (Tribasic) : 0.0358 gm, Glucose : 0.6000 gm, and Distilled water : 1000 ml ) The pH of the solution was adjusted to 7.7 by adding 10-15 ml. of pH 7.7 tribuffer. Suitable amounts of glucose were added to the ringer just before use. The ringer when kept in cold could be used up to 15 days. This ringer was found quite satisfactory and the heart maintained a constant beat for considerable time. Frequent changing of the fluid also enhanced the viability of preparation.
METHOD OF DISSECTION
The limbs having been removed, an opening is made on the dorsal aspect of the cephalo-thorax and a large circular portion above the cardiac region excised with the aid of bone forceps, care being taken to separate the chitinous covering from the subjacent pigmented dermis. The latter is then dissected off, the roof of the pericardium removed, and the pericardial cavity flushed out with the perfusing fluid (double distilled water). The heart beat was seen visually. The stop watch was switched on for a minute and the heart beat was noted as control.
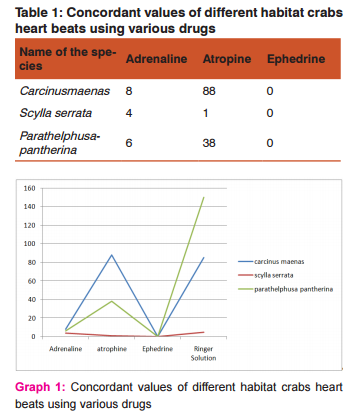
A syringe was loaded with the chemical Adrenaline and poured over the heart of the dissected animal. The stop watch was switched on again and the number of beats per minute was noted. Two more reading were taken and average value of three observations was used for calculating the rate of heart beat expressed in terms of No. of heart beats/seconds. The same procedure was repeated for Atropine, Ephedrine and Ringer solution. The heart beat was tabulated and the concordant values were found for each chemical separately. The rate of heart beats was plotted in relation to time exposure in four chemicals like Adrenaline, Atropine, Ephedrine and Ringer solution. The plotted graphical interpretation has been given the nature of chemical and their intensity and finally the hearts of all treated animal were subjected to histolopathology and haemolymph preparation.
HISTOPATHOLOGY
SOLUTIONS USED
10% Neutral Buffered Formalin and Bouin’s Solution - Fixation: Bouin’s Solution fixed tissue sections must be thoroughly washed in running tap water until all the yellow color (the picric acid) is removed from the tissue. Failure to remove the Bouin’s Solution can result in inadequate staining. Staining problems have been particularly noticed in Immuno-histochemistry staining. Xylene: Deparaffinization : Removal of paraffin from tissue sections. Clearing: Removal of alcohol from tissue section (miscible with permount for coverslipping) 100% Alcohol, Reagent or Absolute (200 proof): Hydration: Removal of xylene from tissue sections and down a gradual series to distilled water. De-hydration:Removal of water from the tissue sections through a graded alcohol to xylene.
95% Alcohol
Alcohol, Reagent 95.0mls 50.0mls 190.0mls Distilled Water 5.0 mls 950.0 ml 3610.0 mls


METHODS: TISSUE ACQUISITION AND PREPARATION:
Heart was carefully removed from three kinds of crabs and placed in a labeled specimen cup containing approximately 75 mls of 10% Formaldehyde in phosphate buffer. The tissues are allowed to fix overnight at room temperature before they are handled. The following day, the tissues are examined and the heart is bisected before all three tissues are placed in labeled cassettes prior to automated processing. They are then bisected at their mid-point to insure that sections will be taken from an anatomically consistent level. The cassettes are returned to the 10% Formaldehyde solution and are held under house vacuum for a minimum of three days until they are ready to be loaded into the automatic tissue processor.
TISSUE PROCESSING: The cassettes are placed in the Microm HMP 300 and processed at room temperature, except where noted, according to the following automated protocol:
Step 1: Fixed in 10% Formaldehyde for 1 hour.
Step 2: Fixed in 10% Formaldehyde for 1 hour.
Step 3: Dehydrated in 50% Ethanol for 45 minutes.
Step 4: Dehydrated in 70% Ethanol for 45 minutes.
Step 5: Dehydrated in 95% Ethanol for 45 minutes.
Step 6: Dehydrated in 95% Ethanol for 45 minutes.
Step 7: Dehydrated in 100% Ethanol for 1 hour.
Step 8: Dehydrated in 100% Ethanol for 1 hour.
Step 9: Cleared in Xylene for 1 hour.
Step 10: Cleared in Xylene for 1 hour.
Step 11: Infiltrated with Tissue Prep II paraffin for 1.5 hours at 60°C, under vacuum.
Step 12: Infiltrated with Tissue Prep II paraffin for 2.5 hours at 60°C, under vacuum.
Step 13: Infiltrated with Tissue Prep II paraffin for 4.0 hours at 60°C, under vacuum.
TISSUE SECTIONING: Three-micrometer thick sections are cut from each tissue on a Microm HM355S microtome. Six slides are produced for each tissue. Tissue sections are mounted on silanized/charged slides with the exception of one uncharged slide dedicated to the Jones Silver Stain. Heart slides carry three section. One slide is stained with Gomori’s One-Step Trichrome for immediate documentation. The rest are held in reserve for future investigation.
TISSUE STAINING: Once all the samples are cut, one slide from each tissue is processed according to this procedure.
Note: Steps 1-10 and 21-28 are performed on the Sakura DRS 2000 Automatic Stainer.
Deparaffinization
1. Xylene, 5 minutes
2. Xylene, 4 minutes
3. Xylene, 3 minutes
4. Xylene, 2 minutes Hydration
5. 100% Alcohol, 2 minutes
6. 100% Alcohol, 2 minutes
7. 95% Alcohol, 1 minute
Rinsing
8. Distilled Water, 1 minute
9. Distilled Water, 1 minute
10. Distilled Water, 1 minute
11. Remove slides from the Sakura Stainer and place in a Tissue Tek ‘White’ staining dish containing fresh distilled water.
Pre-treatment - Mordant: Mandatory: Under a Hood
12. Place slides in the pre-warmed 60 °C Bouin's Solution and incubate at 600 C for 60 minutes (Optional: Bouin’s Solution overnight at room temperature)
13. Remove slides from Bouin’s and place in tap water.
Washing
14. Wash well in running tap water until all the yellow color is removed from the tissue sections.
15. Rinse thoroughly in several changes of Distilled Water and remove slides.
Nuclear Staining
16. Place slides in the ‘Working Weigert’s Iron Hematoxylin Solution’ for 20 minutes. Agitate slides several times during the 20 minutes. Remove slides.
17. Wash well in running tap water until the water runs clean of excess hematoxylin.
18. Rinse thoroughly in several changes of Distilled Water and remove slides. Trichrome Staining
19. Place slides in the room temperature One-Step Trichrome Stain for 45 minutes. Remove slides.
20. Rinse slides thoroughly in several changes of 0.5% Acetic Acid. Agitate slides to remove excess Trichrome Stain. Total time should be approximately 3 minutes. Do not rinse in Distilled Water.
Dehydration
21. Transfer slides to the SAKURA DRS2000 Automated Stainer. Use programmed Staining Method, '95% start TRICHROME’.
22. 95% Alcohol, 45 seconds
23. 100% Alcohol, 1 minute
24. 100% Alcohol, 2 minutes
Clearing
25. Xylene, 3 minutes
26. Xylene, 4 minutes
27. Xylene, 5 minutes
28. Xylene, End Station
Coverslipping / Mounting
29. Remove the slides from the SAKURA stainer and place the staining rack in a green chemical resistant Tissue-Tek staining dish filled with Clear-Rite 3.
30. From Clear-Rite 3 coverslip using Permount and appropriate sized coverglass.
31. Label slides if necessary and arrange accordingly.
PREPARATION OF HAEMOLYMPH SMEAR
A grease free glass slide was labeled on one edge and marked with the sample name. Marking the slides makes it convenient to identify the surface of the slide that contains the smear. Take a live healthy specimen and cut the chelate leg carefully. Place two to three drops of haemolymph on one corner of the slide. Place another clean glass slide at an angle of 450 and pull the slide to another corner of the slide to make a thin smear. Presence of grease on the slide would prevent uniform distribution of the sample resulting in an uneven smear. Taking too much of the sample would result in excessively thick smear. Similarly, taking a very miniscule part of the sample make it very difficult to look for cells. The smear was air dried and heat fixed using a Bunsen flame at a comfortable height.
The smear must not be forcibly dried by applying heat. It is convenient to heat fix the smear by gently passing the slide through the Bunsen flame once or twice. Excessive heating of the slide must be strictly avoided. Fixed smears were placed on a staining rack over a sink or other suitable receptacle. Smears were stained easily separately with any of the stains like alkaline methylene blue for 1 to 15 minutes; with Carbolfuchin for 5 to 10 seconds; with Crystal violet for 20 to 30 seconds; with Safranine for 1 minute. Stained smears were washed gently in running tap water for a few seconds. Blot the slides dry with filter paper. Be careful not to rub the smear when drying the slide because this will remove the stained cells. Stained smears were air dried completely.
Add 1 to 2 mls of the DPX mount on the air dried slides containing the smears using a glass rod. Place the slides on a hot plate for a few minutes till the DPX mount begins to fume. As soon as mount begins to fume, remove the slides from the hot plate and place thick glass cover slips over the slides. Invert the slides so that the mount easily spreads over the slide. Gently press the cover slips with another slide. Do not press too much as it causes damage to the cover slips. Air dry the mount preparations and observe under 100X oil immersion objective.
DOCUMENTATION Using a Nikon E-400 fitted with a Spot Insight camera, six high-resolution color digital micrographs are taken for each of the three crabs per strain. (Three for the heart) The micrographs are taken following a strict protocol that insures that valid comparisons can be made across strains. After all the crabs are documented, half of them will have their images processed into smaller JPEG files and cataloged for posting on the website. This results in a representative survey of the morphology of three tissues from three treatment protocols as expressed within a strain.
RESULTS The circulatory system is the communicating organ system as it communicates with various systems of the body. It ensures proper distribution of oxygen and nutrients apart from the collection of metabolic wastes and delivering to the excretory organs like kidney. The circulatory body fluids transport various chemical substances necessary for metabolic continuity like hormones etc., the system of structures consisting of the heart, blood and blood vessels is named as circulatory system. In these animals the system of cavities should be considered as haemocoel and the blood which fulfils the characters of a circulatory fluid and these of inertial lymph should be caused haemolymph.
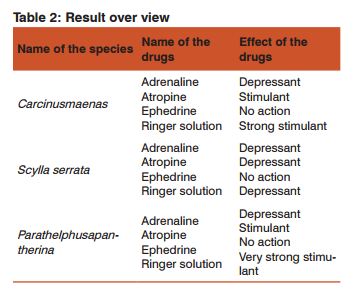
The pigment haemocyanin is found in the blood of most crustaceans, hence it is light blue in colour. The marine crab Carcinusmaenas, the estuarine crab Scylla serrata and the freshwater crab Parathelphusapantherina were selected for experimentation. The animals were collected and brought to the laboratory for acclimatization. The animals were dissected and heart of the animal has been subjected to the effects of some common drugs like Adrenaline, Atropine and Ephedrine. The observations indicate that the drugs like Adrenaline and Atropine stimulate the heart and hence increase in the heart beat whereas Ephedrine inhibited the heart beat or showed no action on the heart.
Similarly, the crabs were subjected to the effects of Ringer solution. It was observed that the toxic effect of the Ringer solution disturbs the regular working of heart and results in uncountable heart beats. The hearts of the three different crustaceans were isolated for the histopathology process to study, diagnose and to differentiate between the cardiac muscles of the crabs. It was observed that edema was seen in all the smears but it was higher in marine species than the estuarine and freshwater species. Vascular degeneration was observed in all the species but it was higher in estuarine crab than the other crabs. Inflammation was also seen but except marine species, the others show inflammation.
There were no toxic accumulation and pathogens observed. Haemolymph from all the three crustaceans were collected and made into smears. Unlike human blood, haemolymph of crustaceans is less denser and light blue in colour. This colour is due to the presence of the pigment named, haemocyanin. Since haemolymph is the circulatory fluid of the crabs, any drug that added to the heart easily communicates with the entire circulatory system and also with the nervous system. It was observed that the smears were even and contains many cells which are irregular in their shape and size. Some of the cells were stained pale blue in colour and some are darkly stained. Some cells were found in groups. There were some nucleated cells observed here and there among the other cells.
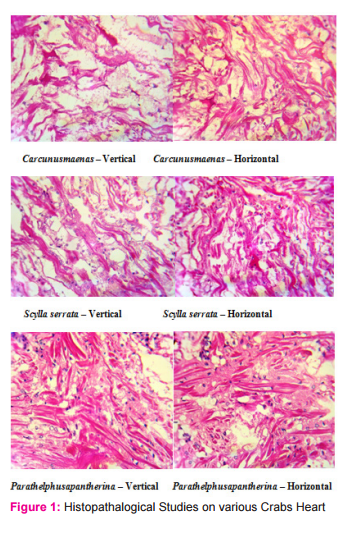
DISCUSSION
The available literature reveals that the heart rate in crustaceans influenced by a number of factors like body size, activity, respiration, stress, light, blood, moulting, diurnal cycle, PH etc. The heart beat frequency has been studied with a conclusion that alterations in heart rate occurs readily with change in environment and have an impact upon heart rate of marine and aquatic crabs. Depletion in the rate of oxygen consumption upon exposure to heavy metal stress weight be due to a penetration of the pollutants at sub-cellular levels and damage of gill tissue, thereby failure of an alternative compensatory mechanism to achieve energy generation for combating toxic stress. Present study was under taken to study the effect of chemicals such as Adrenaline, Atropine and Ephedrine on cardiac physiology of crabs Carcinusmaenas, Scylaserrata and Parathelphusapantherina.
The obtained results clearly indicates that the effects of chemicals i.e., Adrenaline, Atropine and Ephedrine initially inhibit the heart rate which indicates animal try to settle-down into the chemical medium but later on the effects of toxicants accelerate the metabolic activity of the animals which accelerate the rate of heart beat in a crab after this treatment. Then the heart rate slowly decreases as the animal dies. Haemolymph contains haemocyanin, which is oxygen binding site in blood. It is known, that the frequencies of crustaceans heart rate differ under different environmental conditions. It varies not only dismal rhythm, but also lie to temperature, hydrogen ion concentration, toxicants, sex and size. Hence recording time of observations and other factors were also taken into consideration and kept constant.
Thus any environmental factor that alters the process of oxygen uptake can be expected to affect circulation. Of course it is not exclude the possibility of some chemical acting directly on the heart and blood vessels. Crustaceans possess an open type of circulatory system and the haemolymph flows in the blood sinuses. A dorsally situated heart is present in most crustaceans. Though a true heart is lacking in Cirripedes and many Copepods and Ostracodes (Lockwood, 1968). The brachyuran heart is rhomboidal in shape and helps in circulating the haemolymph in the body with the help of its rhythmic beating (Lock Wood, 1968).
Crustaceans are the best studied invertebrate among which freshwater crabs have been intensively investigated with reference to their physiological aspects (Maynard, 1960; Vasantha and Gangotri, 1979). The data which available on the rate of heart beat in a variety of crustaceans list it is influenced by several factors of which size (Posser, 1973). As per the general rule it might be stated the heart rate varies inversely with body size and the trend is valid for most of the crustaceans. Blood volume has been determined in a number of crustaceans including crabs and it is evident that it is highly variable(Maloeuf, 1939; Schwarzkopf, 1955), studied different heart rates in a variety of crabs of different body weights and he showed clearly that the heart rate decreases in heart exponentially with increasing body size.
Environmental pollutants brings about the damage to different organs of disturb the physiological and biochemical processes of the organism following exposure to pollutant. Effects of different pesticides, inorganic ions, drugs and antibiotics on crustaceans hearts specially crabs have been used by many workers to reach the details about adrenergic and cholinergic property of crustacean heart (Agrawal et al., 1965; Bain, 1929; Davenport, 1941). Some important contributions have been made regarding cardio-vascular system by Cameron (1975) in land crab Gecarcinuslateralis. Likewise some other (Ashsanullah and Newell, 1971; Taylor et al., 1973; Hume and Belind, 1976) have been studied about cardiovascular system in Carcinusmaenas.
The hearts of various invertebrates studied differ greatly in their sensitiveness to the action of the drugs. The action of the alkaloids on the heart when introduced into the intact animal is therefore complicated by their action on the central ganglia or brain and on the peripheral ganglia other than those in the heart. The solutions of the alkaloids may be applied to the surface of the excised heart and empty heart or the heart may be filled with the solution. The acceleration of rhythm is followed by depression and if the concentration of the alkaloids is great, by complete cessation of the rhythm, the heart remaining excitable to direct stimulation. The point of action of the alkaloids in the heart is not yet known.
On the myogenic theory of heart beat their stimulating effects may be due to action on the accelerator nervous mechanism or to a direct action on the heart muscle. On the neurogenic theory the augmentation of the rate of the beats can hardly be accounted for except by direct action on the local ganglia, while the increased amplitude of the contractions may be due to the action on the muscle. To answer the question whether the alkaloids act on the nervous or on the muscular tissue in the heart or on both, several investigators have studied their action on the embryonic heart on the theory that the heart on the embryo begins to beat before any nervous elements are present. Pickering (1893, 1894- 95) found that Atropine and other alkaloids accelerate the embryonic heart, while strong solutions of Atropine depress the rhythm without any primary stimulation.
Cyrillo (1901) states that Atropine depresses the embryonic heart. This investigator finds, moreover, that the action of the principal alkaloids on the embryonic heart is the same as on the heart of adults, from which he concludes that these drugs act primarily on the heart muscle, their action on the nervous tissue in the heart being entirely of a second character. But Carlson proposed opposite conclusion that the primary action of the alkaloids is on the ganglion cells in the heart and not on the muscle. This conclusion is based on the results on the heart of Limulus. Cardiac muscle (heart muscle), like skeletal muscle, is also striated but involuntary muscle responsible for the pumping activity of the vertebrate heart. The individual muscle cells are joined through a junctional complex known as the intercalated disc and are not fused together into multinucleate structures as they are in skeletal muscle. Though unlike skeletal, cardiac muscle cells are short and branched with a single, centered nucleus.
They are also involuntary or not under immediate conscious control. Rather than Z-disks, which join skeletal muscle cells, intercalated disks join cardiac muscle fibers. Cardiac muscles are located only in the heart. Unlike skeletal, cardiac muscle can contract without extrinsic nerve or hormonal stimulation. It contracts via its own specialized conducting network within the heart, with nerve stimulation causing only an increase or decrease in rate of conducting discharge. The heart also has some very beneficial features such as an increased number and larger mitochondria, which allow it to produce more ATP. This is very important since the heart is constantly contracting and relaxing. Cardiac muscle can also convert lactic acid produced by skeletal muscle to ATP.
This is quite ingenious since lactic acid is a by-product of muscle when in a deoxygenated state, a state that would be detrimental to cardiac muscle. This muscle also remains contracted 10 to 15 times longer than skeletal muscle due to a prolonged delivery of calcium. Likewise, it also has a relatively long refractory period, lasting several tenths of a second, allowing heart to relax between beats. This also allows heart rate to increase significantly without causing it to go into tetanus, which would be fatal since it would cause blood flow to cease. Crustaceans possessing many primitive features tend to have myogenic hearts, although neurogenicity is dominant in the more advanced malacostracan groups and possibly members of the Ostracoda.
Exactly how the heart is regulated, via neuronal and neuro-hormonal controllers, has recently been investigated and there is a long history of examining the effect of environmental factors (e.g., hypoxia, salinity) on aspects of cardiac function (McMahon, 1999a, 2001; De Pirro et al., 1999). In particular the advent of non-invasive techniques to measure heart rate has opened up possibilities for more realistic measurements both in the laboratory and the field (e.g., Paul et al., 1997; Lundebye and Depledge, 1998; Bloxham et al., 1999).
CONCLUSION This report will be the basis for the future studies on different crustaceans and molluscans. In attempt to evaluate the effects of various drugs on different habitat crabs, we conclude that that the drugs like Adrenaline and Atropine stimulate the heart and hence increase in the heart beat whereas Ephedrine inhibited the heart beat or showed no action on the heart. Similarly, the crabs were subjected to the effects of Ringer solution. It was observed that the toxic effect of the Ringer solution disturbs the regular working of heart and results in uncountable heart beats and haemolymph of crustaceans is less dense.
ACKNOWLEDGMENT Research author was very gratefully thanks to my Research Supervisor Dr. J.M.V. Kalaiarasi and members from the School of Environmental Toxicology and Biotechnology, Department of Advanced Zoology and Biotechnology, Loyola College, Chennai for their cooperation for preceding the research works and for encouraging me to perform the research. Authors are grateful to IJCRR editorial board members and IJCRR team of re-viewers who have helped to bring quality to this manuscript.
References:
1. P. Tyagi, Cardiac pharmacology of the freshwater crab Paratelphusamasoniana, (Henderson) [Department of Zoology, D. A. V. College, Muzaffarnagar (U. P), India, Received January 20, 1970]. (Communicated by Dr. B. S. Chauhan, F. A. Sc.)
2. Aidan J. Mullen GRSC MICI, The synthesis and pharmacology of ephedrine and analogues, Dublin City University, September 1991.
3. Aruna More, Sub-lethal effects of copper sulphate, zinc sulphate and cadmium sulphate on the rate of heart beat of freshwater crab Barytelphusaguerini, Scholars research library, annals of biological research, 2011, 2(6): 437-440
4. Barde R. D, Cardiac physiology of freshwater male crab, Barytelphusaguerini under sumidon and acephate stress, Department of Zoology, S. G. B. S. College, Purna, Biolife,2014, Vol 2, Issue 4.
5. Brian R. Mc Mohan, Control of cardiovascular function and its evolution in crustacea, The journal of experimental biology 204, 923-932,2001.
6. Damian Oliva, Violeta Medan and Daniel Tomsic, Escape behavior and neuronal responses to looming stimuli in the crab Chasmagnathusgranulatus (Decapoda: Grapsidae), 2007, The journal of experimental biology 210, 865-880
7. Deshai R. B, Shinde C. D, Katore B. P and Ambore N. E.The lethal effect of dimethoate on heart beat rate of female crab Barytelphusaguerini, journal of experimental sciences, 3(8): 04-06, 2012.
8. E. R. Baylor, Cardiac pharmacology of the cladoceran, Daphnia, Department of Zoology, University of Illinois, Urbana,165-172.
9. Eyal Robenshtok MD, Shay Luria MD, Zeev Tashma PhD and Ariel Hourvitz MD, Adverse reactions to atropine and the treatment of organophosphate intoxication, Israel Defense Forces Medical Corps, IMAJ Vol 4, July 2002
10. G. Guerao, P. Abello and J. Cartes, Morphology of the megalopa and first crab instar of the shamefaced crab Calappagranulata (Crustacea, Brachyura, Calappidae), Miscel-laniaZoologica 21.1 (1998)
11. G.D. Stentiford, S. W. Feist, A histopathological survey of shore crab (Carcinusmaenas) and brown shrimp (Crangoncrangon) from six estuaries in the United Kingdom, (Received 14 July 2004: Accepted 3 January 2005), ELSEVIER Journal of Invertebrate Pathology 88 (2005) 136-146
12. Guoyi Ma, Supriya A. Bavadekar, Yolande M. Davis, Shilpa G. Lalchandani, Rangaswamy Nagmani, Brian T. Schaneberg, Ikhlas A. Khan, and Dennis R. Feller, Pharmacological effects of ephedrine alkaloids on human α1- and α2- adrenergic receptor subtypes, The journal of pharmacology and experimental therapeutics(Received January 29, 2007; accepted April 2, 2007). 214-221,2007.
13. Hiroshi Ando and Kiyoakikuwasawa, Neuronal and neurohormonal control of the heart in the stomatopod crustacean, Squillaoratoria, 2004.The journal of experimental biology 207, 4663-4677.
14. J. S. Alexandrowicz and D. B. Carlisle, Some experiments on the function of the pericardial organs in crustacea, The Plymouth Laboratory, JOURN. MAR. BIOL. ASSOC. Vol. XXXII, 1953
15. Jennifer L. Martin, Jake Begun, Michael J McLeish, Joanne M. Caine and Gary L. Grunewald, Getting the adrenaline going: Crystal structure of the adrenaline-synthesizing enzyme PNMT, October 2001, Volume 9, Issue 10, pages 977-985.
16. John G. Milton and Mony M. Frojmovic, Montreal, Quebec, Canada.Adrenaline and adenosine diphosphate-induced platelet aggregation require shape change, importance of pseudo-pods, 1984. The journal of laboratory and clinical medicine, St. Louis, Vol.104, No. 5.,805-815.
17. John H. Welsh, Chemical mediation in crustaceans. I. The occurence of acetylcholine in nervous tissues and its action on the decapod heart. Marine Biological Laboratory, Plymouth, Zoological Laboratory, Cambridge University, and Biological Laboratories, Harvard University.JEB.XVI ii.
18. K. K. Chen and Carl F. Schmidt, Ephedrine and related substances, Medicine monographs, Department of pharmacology, Vol XVII, 1930.
19. Lancelot T. Hogben, M.A., D.Sc., Department of Physiology, Edinburgh University, and A. D. Hobson, B.A., Department of Zoology, University College, London, Studies on internal secretion III- The action of pituitary extract and adrenaline on contractile tissues of certain invertebrata, 1924. Vol I, no.4.
20. Michael W. Owens, M.D., F. C. C. P., Ronald B. George M.D., F. C. C. P., Nebulized Atropine sulfate in the treatment of acute asthma, Chest 1991; 99; 1084-87.
21. P. M. M. Schuwerack, J. W. Lewis and P. W. Jones, Pathological and physiological changes in the South African freshwater crab Potamonauteswarreni Calman induced by microbial gill infestations, 2001,Journal of invertebrate pathology77, 269-279.
22. Richard J. Wurtman, Julius Axelrod, Adrenaline synthesis: Control by the pituitary gland and adrenal glucocorticoids, National Institute of mental health, December 10, 1965, Vol. 150, No.3702, pages 1464-1465.
23. Richard Pearse Drive, Aspen adrenaline injection, data sheet, Pharmacy retailing (NZ) limited, 29 april, 2013.
24. Soundarapandian P., Ravichandran S and Varadharajan D., Biochemical composition of edible crab, Podophthalmus vigil (Fabricus), JMSRD, Marine Science, Research and Development, 2013. An open access journal, Volume 3, Issue 2
25. Tucson, Ariz., Reports on real risk of errors in tissue biopsy processing, Journal of Histotechnology, Ventana medical systems, Roche group, July 18, 2013.
26. W. A. Bain, The action of adrenaline and of certain drugs upon the isolated crustacean heart, From the Marine Biological Laboratory, Plymouth and the Department of Physiology, University of Edinburgh, (Received for publication 15th January 1929)
27. W. J. Crozier and T. J. Stier, Temperature characteristics for heart rate in embryos of Limulus, The society for experimental biology and medicine, 1927, XXIV, 339-340.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License