IJCRR - 13(7), April, 2021
Pages: 142-148
Date of Publication: 12-Apr-2021
Print Article
Download XML Download PDF
Anti-inflammatory Activity of Aerial and Root Extracts of Withania somnifera: A Comparative Experimental Evidence
Author: Satyajyoti Kanjilal, Ashok Kumar Gupta, Ranjana Saksena Patnaik, Amitabha Dey
Category: Healthcare
Abstract:Introduction: Withania somnifera Dunal (Ashwagandha) root is recognized as one of the medicinally important herbs for its anti�inflammatory properties. Comparative study on both the parts i.e. aerial and root has not been studied yet from the perspectives of its anti-inflammatory activities. Objective: The present study aimed to evaluate the comparative anti-inflammatory activity of standardized methanolic and hydro-methanolic extract of both the roots and the aerial part of the plant in in-vitro models of TNF-\a, Interleukin-1 beta and IL-6. Methods: Hydroalcoholic and methanolic extracts of Withania somnifera aerial part and root part were prepared. Respective as�says were performed to estimate the cytokines by using Elisa Kit and absorbance was noted in the Multimode Microplate reader. Results: Both the methanolic and hydroalcoholic extracts from the aerial part was found to have more potential compared to that of roots in inhibiting the TNF \?alpha cytokines and Interleukin 6 inhibition assay. Root Hydroalcoholic extract was better than aerial methanolic extract. In the interleukin beta inhibition assay, both root extracts were better than aerial extract. Conclusion: Both root and aerial parts of Withania somnifera possess anti-inflammatory properties. The aerial part has shown a promising activity vis-à-vis the roots. Therefore, this can be an alternative renewable resource as raw material for preparing medicines for arthritis.
Keywords: Ashwagandha, Inflammation, Interleukin, TNF alpha, Withania somnifera
Full Text:
Introduction
Cytokines are small-secreted proteins referred inconsistently as interleukins, growth factors or chemokines. Physiologically, they activate the inflammatory mechanisms and help repair damaged tissue. During the inflammatory process, a sudden release of cytokines (cytokine storm) occurs via a cascade of activation process.1,2 The uncontrolled expression of these macrophage-derived cytokines (inflammatory mediators) tumour necrosis factor (TNF), interleukins (IL-1, IL-6, IL-8), colony-stimulating factors (CSFs) and growth factors govern the pro-inflammatory signalling pathways in the pathogenesis of rheumatoid arthritis (RA).2,3 The activated macrophages produce TNF-α in the inflamed synovial membrane tissue. This is having the capacity of induction and production of other pro-inflammatory cytokines, including Interleukin-1 beta (IL-1β) and IL-6. Combined facilitation of signalling pathways and cytokines, in turn, release chemokines that attract leukocytes to the inflamed site from the blood. The induction of proteolytic and metalloproteinase enzymes further results in the destruction of the underlying articular cartilage and bone tissue.4-10
The mainstay of the treatment of RA is to control these pro-inflammatory mediators. At therapeutics of Disease-Modifying Antirheumatic Drugs (DMARDs) such as Sulfasalazine, Methotrexate, Leflunomide, Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) and biologics such as Infliximab, Adalimumab (TNF –blocker), Anakinra (IL-1 blocker), Tocilizumab (IL-6 blocker) are been looked upon for the management.4,11
The drug regimen offers efficient management, however, the chance of suboptimal control, the inability to complete remission and emerging adverse effects in long-term usage often lead to a search for more alternatives.12-16 The need-gap analysis and increasing popularity of plant or herb-based medicine often push for innovations and research which lead to the discovery of many new phytomedicines or enriched extracts or drugs.17 Many plants and formulations are reported to have anti-inflammatory and analgesic properties.18-20
Withania somnifera Dunal (Ashwagandha) or Indian ginseng has been recognized as one of the medicinally important herbs having anti-inflammatory and Rasayana (adaptogen and rejuvenator) properties. Among the other phytoconstituents, the biologically active steroidal lactones Withaferin A and Withanolides were reported to have anti-inflammatory and immunomodulatory properties contributing to their use in painful arthritic conditions.21 Roots are researched for their potent inhibitory effect on inflammatory markers and used in traditional system of medicine.21-23 The aerial part especially the leaves of the plant are also studied for its role as anticancer, antimicrobial, diabetes etc. However, a comparative study on both parts especially the commercially viable extracts are not been studied in the anti-inflammatory perspectives. Therefore, the present study was conducted to evaluate the comparative anti-inflammatory activity of standardized methanolic and hydro-methanolic extract of both the roots and the aerial part of the plant in in-vitro models of TNF-α, Interleukin-1 beta (IL-1β) and IL-6.
Materials and Methods
Plant Material
Withania somnifera raw material of aerial part Lot No.:ASHW/PLPLR30/NOV19 and Aswagandha aerial part hydroalcoholic extract 5%, Lot No: ASHW/PLPL30/NOV were procured from Phyto Life sciences Pvt Ltd, Ahmedabad, Gujarat. The root powder was obtained from Emami Ltd, Research and Development Centre, Kolkata. Both the raw material of aerial and root were further used to get the methanolic and hydroalcoholic extracts in the phytochemistry laboratory of Emami Research and Development Centre.
Chemical and Reagents, Kits, Drugs and Cell line
Reagents and Kits: The TNF alpha Human ELISA Kit (Abcam; Cat. No. ab100654), ab46052 IL-1 beta Human ELISA Kit and ab46042 IL-6 (Interleukin-6) High Sensitivity Human ELISA Kit of Abcam. Drugs: The Anti-arthritic drugs were purchased from the local pharmacist - Methotrexate 25 mg (Folitrax) Batch No. At 071110, Sulfasalazine (Saaz) Batch No. ECL079007AS marketed by IPCA Laboratories Ltd, Mumbai and Diclofenac (Voveran 50) Batch No. 195009MB of Novartis India Ltd were also used. Cell culture mediums and Solutions: Dulbecco's Modified Eagle's medium (DMEM), Fetal Bovine Serum (FBS) & 1% Antibiotic/anti-mitotic solution. MTT (3-(4, 5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) reagent (HiMedia). Dulbecco's phosphate-buffered saline (DPBS - HiMedia). Dimethyl sulfoxide (DMSO). All the chemical and drugs are procured from Local vendor. Cell line: MG 62 and Hep was procured from National Centre for Cell Science, Pune, India.
Preparation of W somnifera extracts
Alcoholic and hydroalcoholic extracts of roots and leaf part of Withania somnifera were evaporated using a rotary vacuum evaporator, whereas aqueous extracts were dried by lyophilization. HPTLC (high-performance thin-layer chromatography) qualitative studies showed that the aqueous-methanolic extracts of both plant parts contain higher amounts of withanolides than all other extracts concerning withaferin A and withanolide A. Accurately weighed 1.0 g of leaf and root powder was soaked separately in 50 ml conical flask with 20 ml of 50% aq-methanol. Both the samples were sonicated at 60 C for 30 minutes and samples were filtered.
For preparing the reference standard solution, 10.0 mg of withaferin A and withanolide A were dissolved with methanol in 5 ml volumetric flasks which were further made 10-fold dilution. Silica gel aluminium sheet plate 60 F254 (Germany) was used to perform the chromatographic estimation. Linear ascending development was carried out in a twin-trough glass chamber (CAMAG) equilibrated with the mobile phase. The length of the chromatogram run was 6.0 cm. Concentrations of the compound were determined from the intensity of absorbance. The evaluation was made via peak areas versus withanolides amount in linear regression. The withanolides present in the extracts were identified by comparing the HPTLC spectrum of the standards
Cell Line and Culture Condition
The monolayer of the cells was maintained at sub-confluent conditions in growth media containing DMEM with 0.045 g/ml glucose, 1mM sodium pyruvate, L-glutamine, 1.5 g/L sodium bicarbonate, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% fetal bovine serum (FBS). Cells were maintained in a humidified incubator with ambient oxygen and 5% CO2 at 37°C. Cells from passages 3–15 were used in the experiment, and cells were not allowed to grow to more than 60%–70% confluence.
Prepare the cell culture
100 μl of differentiated cell suspension (1 × 106 cells/ml) were seeded in a 96-well culture plate with 100 μl of DMEM – differentiated cell culture medium. The cells were incubated in an FBS-free medium for 4 to 18 hr before the examination.
Effect of test samples on cell viability and proliferation: MTT assay24
Measurement of cell viability and proliferation forms the basis for numerous in vitro assays of a cell population’s response to external factors. The yellow tetrazolium MTT is reduced by metabolically active cells, in part by the action of dehydrogenase enzymes, to generate reducing equivalents such as NADH (nicotinamide adenine dinucleotide hydrogen) and NADPH (nicotinamide adenine dinucleotide phosphate hydrogen). This reduction takes place only when mitochondrial reductase enzymes are active, and therefore conversion can be directly related to the number of viable (living) cells. Sample extracts were prepared in the concentration of 100 to 1000 µg/ml. MTT reagent was prepared aseptically by adding 6 ml of cell-based assay buffer (DPBS) in a vial containing 30 mg of MTT reagent and dissolved completely by vigorous vortexing.
Assay Procedure
The wells for control, samples and blank were designated in 96 well plates. The culture medium from each well was removed and washed the cells twice with DPBS. The test samples were added to the 96 well plate containing cells and incubated in a 5% CO2 incubator at 37°C for 18-20 hrs. (The volume of the test sample solution is 100 μl). After the incubation period, the plates from the incubator were removed and 10 µl of MTT reagent (5 mg/ml) were added to all the wells except blank wells. The culture plates were wrapped with aluminium foil to avoid exposure to light. The plates were returned to the CO2 incubator and incubated for 3 to 4 hours. The culture medium was carefully removed from each well after the process of incubation was over and 100 µl of DMSO were added to each well to solubilize the Formazan crystals. Absorbance was noted on Multimode Microplate reader: SpectraMax i3X [Molecular Devices] at 570nm. The average 570 nm absorbance values of the control wells were subtracted from the average 570 nm absorbance values of corresponding experimental wells.
For optimization of the process, the cell suspension was serially diluted from 1 × 106 to 1 × 103 cells/ml using the appropriate culture medium. 100 µl of each dilution were seeded in a 96-well plate and a curve of absorbance against cell density was plotted. The optimal number of viable cells after treatment with samples are measured from the linear graph.
Estimation of Cytokines – TNF-α
TNF alpha Human ELISA (Enzyme-Linked Immunosorbent Assay) kit is an in vitro enzyme-linked immunosorbent assay for the quantitative measurement of Human TNF alpha in serum, plasma and cell culture supernatants. This assay employs an antibody specific for Human TNF alpha coated on a 96-well plate. All reagents, samples and standards are prepared as per the instructions on the manual of the kit. Standards and samples 100 μL each are pipetted into the wells and TNF alpha present in a sample is bound to the wells by the immobilized antibody. At each well, the following sequence is followed with incubation at room temperature in between after each addition. The wells are washed and 100 μL of 1X Biotinylated TNF alpha Detection Antibody is added. After washing away the unbound biotinylated antibody, 100 μL of 1X HRP-Streptavidin solution is pipetted to the wells. The wells are again washed, and 100 μL of TMB One-Step Substrate Reagent is added to the wells and colour develops in proportion to the amount of TNF alpha bound. 50 μL of Stop Solution is added to each well which changes the colour from blue to yellow, and the intensity of the colour is measured at 450 nm on Multimode Microplate reader: SpectraMax i3X [Molecular Devices, USA]. The calculations were performed as per the Kit protocol.
Estimation of Cytokines - IL-1β
The ELISA (Enzyme-linked Immunosorbent Assay) kit for estimation of human IL 1β is intended to quantify the enzyme in blood components or culture medium. For experimentation, samples 100µL each is pipetted into the specific antibody-coated wells of microtiter strip microplates. To begin the experimental process, samples, as well as standards and 50 µL of 1X Biotinylated monoclonal anti-IL-1 beta antibody, are simultaneously incubated. After washing, the enzyme 100 μL of 1X Streptavidin- HRP, that binds the biotinylated antibody is added, incubated and washed. A 100 μL of Chromogen TMB substrate solution is added which acts on the bound enzyme to induce a coloured reaction product. Direct exposure to light is avoided by wrapping the plate in aluminium foil. 100 μL of Stop Reagent is added into each well and results are taken immediately after the addition of Stop Reagent, or within one hour, if the microplate is stored at 2-8°C in the dark.
The absorbance of each well is read on a spectrophotometer (SpectraMax i3X) using 450 nm as the primary wavelength and optionally 620 nm (610 nm to 650 nm is acceptable) as the reference wavelength. The more is the concentration of the interleukin beta in the sample, the more is the strength of the coloured product. The standardized protocol mentioned in the kit is followed to calculate during experimentation and analysis.
Estimation of Cytokines - IL-6
ELISA kit for Interleukin-6 in-vitro testing is intended to quantify the enzyme in different blood components, body fluids and experimental solutions. The assay method identifies natural as well as recombinant human IL-6. The coating of interleukin-6 monoclonal antibody was done before the experiment in the specified number of microtiter strips well-plates. Each well was filled with standards and control samples in the quantity of 100 μL respectively. The experiment was started with the incubation of 50 μL of 1 X Biotinylated monoclonal anti-IL-6 antibody along with either samples or standard. After washing, 100 μL of 1X Streptavidin-HRP solution, that binds the biotinylated antibody is added into all wells, incubated at room temperature for 30 minutes and washed.
100 μL of Chromogen TMB (3,3',5,5'-tetramethylbenzidine) substrate solution is added which acts on the bound enzyme to induce a coloured reaction product and incubated in the dark for 12-15 minutes at room temperature. The intensity of this coloured product is directly proportional to the concentration of IL-6 present in the samples 100 μL of Stop Reagent is added into each well and results are taken immediately after the addition of Stop Reagent, The absorbance of each well was noted on a spectrophotometer (SpectraMax i3X ) using 450 nm as the primary wavelength and optionally 620 nm (610 nm to 650 nm is acceptable) as the reference wavelength. The calculations were performed as per the Kit protocol.
Statistical Analysis
Mean ± standard error of the mean (SEM) were calculated from the observed individual values Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Student-Newman-Keuls multiple comparison test. GraphPad Prism- 5 (GraphPad Software Inc., La Jolla, California, USA) was used for statistical analysis. P-values less than 0.05 were considered statistically significant.
Results and Discussion
The phytochemical analysis of W. somnifera has revealed several groups of bioactive compounds such as flavonoids, tannin, alkaloids, sitoindosides, glycosides, withanicil, steroidal lactones, and alkaloids.25,26 The most therapeutically important chemicals are withanolides especially withaferin A and withanolide D for its antiarthritic effects.27,28 Compounds in sample chromatograms were determined from the intensity of absorbance at 232 nm and identified invalidate High-performance thin-layer chromatography (HPTLC) analysis, which is an efficient tool for quantitative analysis of compounds and yields superior separation efficiency. The withanolides present in the extracts were identified by comparing the HPTLC spectrum of the standards. The quantities of withanolides were present more in 50% aq-methanolic extracts of the root part of Ashwagandha.
The inflammatory mediator Tumour Necrosis Factor-alpha (TNF-α) may lead to the release of other inflammatory cytokines such as IL-1β and IL-629, which promotes synovitis causing cartilage destruction and bone erosion in RA.30,31 W. somnifera is a herb known to have multiple benefits to keep the body healthy. The roots of the plant have known usage for having anti-inflammatory properties. Both the Leaf and root extracts of W. somnifera has been studied for analysis and standardization of Phyto-constituents and in efficacy models.32-34
The present study gave a preliminary research lead on the most effective part extract in inflammations in-vitro cytokine models. Many researchers in experimental (in-vitro and in-vivo) anti-inflammatory models have studied the leaf part. W. somnifera leaf aqueous extract and one of its active chloroform fractions studied in microglial cell lines was found to inhibit the microglial activation and migration by attenuating the pro-inflammatory markers of TNF-α, IL-1β, IL-6 and others suggesting its role in suppression of neuroinflammation.35 New withanolide glycosides and withanolide isolated from the leaves were studied for their inhibition of cycloxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) enzymes and lipid peroxidation. The majority of compounds showed selective COX-2 enzyme inhibition suggesting their anti-inflammatory activity.36 The hydro-methanolic extract in stainless steel implant induced inflammation in zebrafish in-vivo model has shown an inhibitory effect of TNF α.37
The root part of W. somnifera finds its usage more in experimental models as well as in clinical practice. Hydroalcoholic root extract showed anti-inflammatory effect by inhibition of protein (albumin) denaturation in the in-vitro model.38 Ethanolic extract was effective in acute and chronic in-vivo carrageenan-induced anti-inflammatory model.39 Aqueous extracts in gel dosage form has shown anti-inflammatory activity in trinitrobenzene sulfonic acid-induced in-vivo models for inflammatory bowel disease.40 Root powder41 and aqueous extract 42 decreased the arthritis effects in collagen-induced arthritis in-vivo model suggestive its anti-inflammatory activity. Attenuation of the pro-inflammatory markers (TNF-α, IL-1β, IL-6 and IL-10) were observed in the study by khan et.al. Root powder showed inhibitory effect on pro-inflammatory markers, proteinuria, nephritis in pristane-induced Lupus model.43 Root power exhibited anti-inflammatory activity by decreasing the pro-inflammatory markers TNF-α and IL-6 in fructose-fed rats44 and showed potent analgesic and antipyretic effect by retarding amplification and propagation of the inflammatory response in monosodium urate crystal-induced (Gout) model.45 Root powder had potent inhibitory activity towards the complement system, mitogen-induced lymphocyte proliferation and delayed-type hypersensitivity reaction suggestive usefulness in immunosuppression for the inflammatory diseases.46
Aqueous root extract showed anti?inflammatory activity on human osteoarthritic cartilage47 and in the human keratinocyte cell line by inhibiting expression of inflammatory cytokines IL?8, IL?6, TNF?α, IL?1β and IL?12, and promoting the expression of the anti?inflammatory cytokine transforming growth factor (TGF)?β1.48
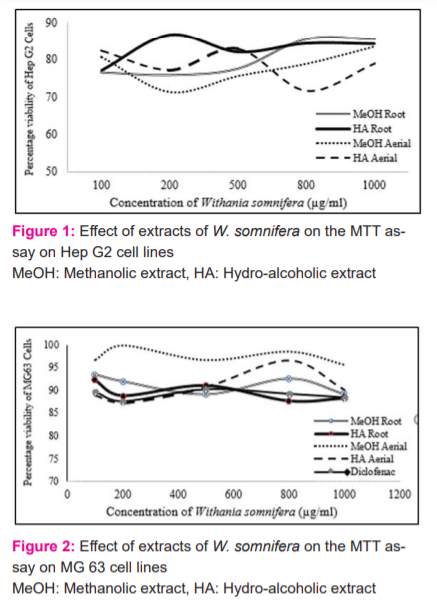
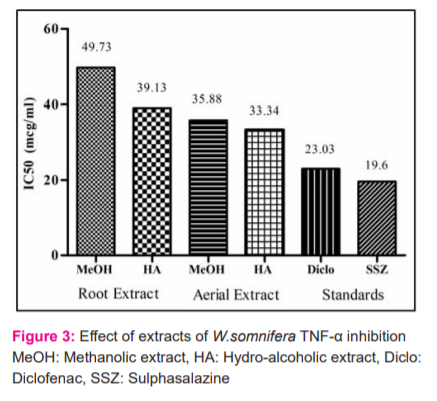
The MTT method of cell determination is useful in the measurement of cell growth in response to mitogens, antigenic stimuli, growth factors and other cell growth-promoting reagents, cytotoxicity studies, and in the derivation of cell growth curves (Sigma). In the present study, the MTT assay was performed in MG 63 and Hep G2 cell line. Methotrexate confirmed the authenticity of the study by showing a decreasing trend in cell viability. All the four extracts from the concentration of 100 µg/ml up to 1000 µg/ml have shown a considerable cell survival count (Cells/ml) contributing towards its safety profile (Figures 1 & 2).
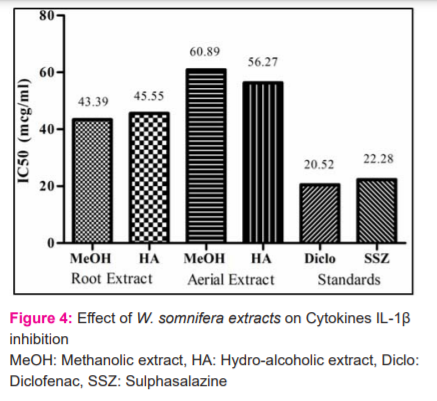
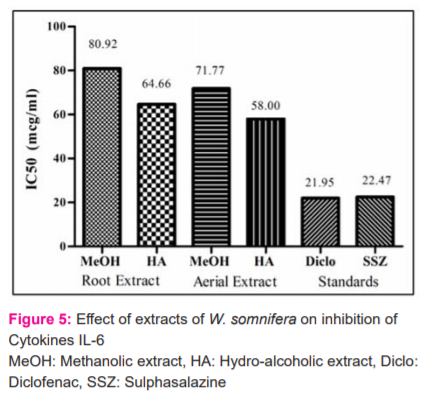
Levels of inflammatory cytokine, TNF-α were measured as a marker of inflammation. Both the methanolic and hydroalcoholic extracts from the aerial part was found to have more potential compared to that of roots in inhibiting the TNF –alpha cytokines (Figure 3). Cytokine IL-1β is reported to play critical roles in the pathogenesis of RA. The inflammatory cytokine IL-6 was measured as a marker of inflammation. In the Interleukin 6 inhibition assay, the extracts of the aerial part were better compared to their respective roots extracts. Root Hydroalcoholic extract was better than aerial methanolic extract. However, among the four, the aerial hydro-alcoholic part is the most potential. In the interleukin beta inhibition assay, both root extracts were better than aerial extract (Figure 4 and 5).
Conclusion
Both parts of W. somnifera possess anti-inflammatory properties however, the aerial part has shown a promising activity vis-à-vis the roots. Therefore, this can be an alternative renewable resource as raw material for preparing medicines for arthritis. Moreover, both the methanolic and hydroalcoholic extracts can be considered for Phyto-pharmaceutical drug development.
Acknowledgements: The authors would like to express their gratitude to the Research and Development centre of Emami Limited, Kolkata, India for providing support for this work. The authors acknowledge the immense help and the knowledge received from the scholars whose articles are cited and included in references to this manuscript. The authors are also grateful to authors/editors/publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
Conflict of interests: Authors declare no conflict of interests.
Funding Source: Nil
Author’s Contribution: SJK and AB have experimented and analysed the data. SJK has prepared the initial manuscript, AKG and RKP have reviewed and modified the manuscript. The manuscript was finalized by SJK and AB after the incorporation of the comments. AKG and RKP has approved the final manuscript.
References:
-
Kay S, Vollrath JT, Relja B. Cytokines in Inflammatory Disease. Int J Mol Sci 2019;20(23):6008.
-
Duff GW. Cytokines and Acute Phase Proteins in Rheumatoid Arthritis. Scand J Rheumatol Suppl 1994;100:9-19.
-
Luo X, Zuo X, Mo, X, Zhou Y, Xiao X. Treatment with recombinant Hsp72 suppresses collagen-induced arthritis in mice. Inflammation 2011;34(5):432-439.
-
Brennan FM, McInnes LB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest 2008;118(11):3537-3545.
-
Feldmann M, Fionula M. Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol 1996;14:397-440.
-
Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet 2010;376:1094-1108.
-
Carson DA, Chen PP, Kipps TJ. New roles for rheumatoid factor. J Clin Invest 1991;87(2):379-383.
-
Komatsu N, Takayanagi H. Inflammation and bone destruction in arthritis: Synergistic activity of immune and mesenchymal cells in joints. Front Immunol 2012;3:77.
-
Matsuno H, Yudoh K, Katayama R, Nakazawa F, Uzuki M, Sawai T, et al. The role of TNF-alpha in the pathogenesis of inflammation and joint destruction in Rheumatoid Arthritis (RA): A study using a human RA/SCID mouse chimaera. Rheumatology 2002;41(3):329-337.
-
Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature 2003;423(6973):337-342.
-
Cronstein BN. The mechanism of action of methotrexate. Rheum Dis Clin North Am 1997;23(4):739-755.
-
Roth SH. Coming to terms with nonsteroidal anti-inflammatory drug gastropathy. Drugs 2012;72(7): 873-879.
-
Schiff M, Keiserman M, Codding C, Songcharoen S, Berman A, Nayiager S, et al. Clinical response and tolerability to abatacept in patients with rheumatoid arthritis previously treated with infliximab or abatacept: Open label extension of the ATTEST Study. Ann Rheum Dis 2011;70(11):2003-2007.
-
Abdel-Tawab M, Werz O, Schubert-Zsilavecz M. Boswellia serrata: An overall assessment of in vitro, preclinical, pharmacokinetic and clinical data. Clin Pharmacokinet 2011;50(6):349-369.
-
Tripathi KD. Essentials of medical pharmacology. 6th ed. New Delhi: Jaypee Brother’s Medical Publishers (P) Ltd.; 2008.
-
Bennett PN, Brown MJ. Clinical pharmacology. New Delhi: Churchill Livingstone; 2005.
-
Katiyar CK, Gupta A, Kanjilal S, Katiyar S. Drug discovery from plant sources: An integrated approach. Ayu 2012;33(1):10-19.
-
. Ismail SM, Leelavathi S, Thara SKJ, Sampath KKK. Evaluation of in-vivo anti-rheumatic activity of Anisomeles malabarica R. Br Int J Curr Res Rev 2012;4(13):118-125.
-
Biswas R, Kanjilal S, Dey A, Mana S, Bhatt B, Pandit S et al. Anti-inflammatory and Analgesic Activity of an Ayurvedic Liniment Formulation. J Drug Res Ayur Sci 2018;3(2):113-118.
-
Dey A, Kanjilal S, Adhikari A, Bhatt BN, Chakraborty T, Chakraborty P, et.al. Anti-inflammatory activity of Zandu Rhumayog Forte and Rhumasyl Gel in acute and chronic inflammatory models. Annals Ayur Med 2019;8(3-4):104-113.
-
Dar NJ, Hamid A, Ahmad M. Pharmacologic overview of Withania somnifera, the Indian Ginseng. Cell Mol Life Sci 2015;72(23):4445-4460.
-
Ayurvedic formulary of India. Part I. 2nd ed. Chapter 8:48 (Vishgarbha Taila). New Delhi, India: Department of Indian System of Medicine and Homeopathy, Ministry of Health and Family Welfare, Government of India;2003.
-
Chunekar KC. Bhavprakash Nighantu (Indian Meteria Medica) of Sri Bhavamishra, Pandey GS (Ed). Varanasi: Chaukhamba Bharati Academy;2015.
-
Freshney RI. Culture of animal cells: a manual of basic techniques. In: Cytotoxicity. 5th edition. New Jersey: Wiley-Liss;2005. p. 359-74.
-
Mirjalili MH, Moyano E, Bonfill M, Cusido RM, Palazón J. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules 2009;14(7):2373-93.
-
Kalra R, Kaushik N. Withania somnifera (Linn.) Dunal: a review of chemical and pharmacological diversity. Phytoche Rev 2017;16:953-987.
-
Ganzera M, Choudhary MI, Khan IA. Quantitative HPLC analysis of withanolides in Withania somnifera. Fitoterapia 2003;74(1-2):68-76.
-
Kumar V, Dey A,Hadimani MB, Markovi? T, Emerald M. Chemistry and pharmacology of Withania somnifera: An update. Humanitas Med 2015;5:1-13.
-
Filippin LI Vercelino R, Marroni NP, Xavier RM. Redox signalling and the inflammatory response in rheumatoid arthritis. Clin Exp Immunol 2008 Jun;152(3):415-422.
-
Hashizume M, Mihara M. The roles of interleukin-6 in the pathogenesis of rheumatoid arthritis. Arthritis 2011;2011:765624.
-
Morel J, Berenbaum F. Signal transduction pathways: New targets for treating rheumatoid arthritis. Joint Bone Spine 2004;71(6):503-510.
-
Chatterjee S, Srivastava S, Khalid A, Singh N, Sangwan RS, Sidhu OP, et al. Comprehensive metabolic fingerprinting of Withania somnifera leaf and root extracts. Phytochemistry 2010;71:1085–1094.
-
Chaurasiya ND, Uniyal GC, Lal P, Misra L, Sangwan NS, Tuli R, et al. Analysis of Withanolides in Root and Leaf of Withania somnifera by HPLC with Photodiode Array and Evaporative Light Scattering Detection. Phytochem Anal 2008;19(2):148–54.
-
Udayakumar R, Kasthurirengan S, Mariashibu TS, Rajesh M, Anbazhagan VR, Kim SC, et al. Hypoglycaemic and Hypolipidaemic Effects of Withania somnifera Root and Leaf Extracts on Alloxan-Induced Diabetic Rats. Int J Mol Sci 2009;10(5):2367-82.
-
Gupta M and Kaur G. Aqueous extract from the Withania somnifera leaves as a potential anti-neuroinflammatory agent: a mechanistic study. J Neuroinflamm 2016;13(1):193.
-
Jayaprakasam B, Nair MG. Cyclooxygenase-2 enzyme inhibitory withanolides from Withania somnifera leaves. Tetrahedron 2003 Feb;59(6):841-9.
-
Sivamani S, Joseph B, Kar B. Anti-inflammatory activity of Withania somnifera leaf extract in stainless steel implant induced inflammation in adult zebrafish. J Genet Eng Biotechnol 2014 Jun;12(1):1-6.
-
Chandra S, Chatterjee P, Dey P, Bhattacharya S. Evaluation of Anti-inflammatory Effect of Ashwagandha: A Preliminary Study in vitro. Pharmacog J 2012;4(29):47-49.
-
Giri KR. Comparative study of anti-inflammatory activity of Withania somnifera (Ashwagandha) with hydrocortisone in experimental animals (Albino rats). J Med Plants Stud. 2016; 4(1):78-83.
-
Pawar P, Gilda S, Sharma S, Jagtap S, Paradkar A, Mahadik K, et al. Rectal gel application of Withania somnifera root extract expounds anti-inflammatory and mucorestorative activity in TNBS-induced Inflammatory Bowel Disease. BMC Complement Altern Med 2011;11:34.
-
Gupta A, Singh S. Evaluation of anti-inflammatory effect of Withania somnifera root on collagen-induced arthritis in rats. Pharm Biol 2014 Mar;52(3):308-320.
-
Khan MA, Ahmed RS, Chandra N, Arora VK, Ali A. In vivo, Extract from Withania somnifera Root Ameliorates Arthritis via Regulation of Key Immune Mediators of Inflammation in Experimental Model of Arthritis. Antiinflamm Antiallergy Agents Med Chem. 2019;18(1):55-70.
-
Minhas U, Minz R, Das P, Bhatnagar A. Therapeutic effect of Withania somnifera on pristane-induced model of SLE. Inflammopharmacology 2012 Aug;20(4):195-205.
-
Noshahr ZS, Shahraki MR, Ahmadvand H, Nourabadi D, Nakhaei A. Protective effects of Withania somnifera root on inflammatory markers and insulin resistance in fructose-fed rats. Rep Biochem Mol Biol 2015 Apr;3(2):62-7.
-
Rasool M, Varalakshmi P. Suppressive effect of Withania somnifera root powder on experimental gouty arthritis: An in vivo and in vitro study. Chem Biol Interact 2006 Dec;164(3):174-80.
-
Rasool M, Varalakshmi P. Immunomodulatory role of Withania somnifera root powder on experimental induced inflammation: An in vivo and in vitro study. Vascul Pharmacol 2006 Jun;44(6):406-10.
-
Sumantran VN, Chandwaskar R, Joshi AK, Boddul S, Patwardhan B, Chopra A, et al. The relationship between chondroprotective and anti-inflammatory effects of Withania somnifera root and glucosamine sulphate on human osteoarthritic cartilage in-Vitro. Phytother Res 2008 Oct; 22(10):1342-8.
-
Sikandan A, Shinomiya T, Nagahara Y. Ashwagandha root extract exerts anti?inflammatory effects in HaCaT cells by inhibiting the MAPK/NF?κB pathways and by regulating cytokines. Int J Mol Med 2018;42(1): 425-34.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License