IJCRR - 8(1), January, 2016
Pages: 25-30
Print Article
Download XML Download PDF
A STUDY OF THE CLINICAL PROFILE OF MALARIA AND ITS COMPLICATIONS
Author: Varsha Shirish Dabadghao, Veer Bahadur Singh, Dayal Sharma, Babu Lal Meena
Category: Healthcare
Abstract:Objectives: Malaria is one of the most widespread infection in the tropics, and also one of the most dangerous. There are four main types of malaria of which Plasmodium vivax and falciparum are common in India. There is a huge burden of disease and malaria is responsible for increased mortality and morbidity. This study was undertaken to evaluate clinical profile of P. vivax, falciparum and mixed malaria and to assess the complications of malaria as a whole and of each type. Methods: This was a cross sectional and observational study which was conducted on a hundred patients above age 14 years diagnosed with malaria. Diagnosis of malaria was made by gold standard method of peripheral blood smear examination and rapid tests. For categorical data, chi- square test was used. P < 0.05 was considered as a statistical significance at 95% confidence intervals. Results: In this study, there were 90% survivors and 10% of patients succumbed. Out of 90 survivors, 43 patients (47.7%) had some form of complicated malaria, whereas all patients who succumbed (10) had complicated malaria. The main complications were severe thrombocytopenia in 78% , significant jaundice (bilirubin> 3mg/dl) in 32% , hypotension with a systolic blood pressure (BP) of less than 70mmHg in 23% , renal impairment in 48% , cerebral malaria in 13%, acute respiratory distress syndrome (ARDS) IN 11%, severe anemia (hemoglobin < 5g/dl) in 10% and metabolic acidosis (bicarbonate < 15mmol/dl) in 5%. Conclusions: The complications seen in this study were mainly severe thrombocytopenia, hypotension, jaundice, renal impairment, cerebral malaria, ARDS, severe anemia and metabolic acidosis; severe thrombocytopenia and jaundice being the commonest, and. P. vivax accounted for more patients with hypotension and severe anemia than the other types of malaria.
Keywords: P. Vivax, P. Falciparum, Malaria, Complications
Full Text:
INTRODUCTION
Malaria is a disease widespread in the tropics, including India. Plasmodium vivax (P. vivax) is the most common prevalent species and Plasmodium falciparum(P. falciparum) the most virulent one worldwide.[1] Malaria is spread primarily by the bite of the female Anopheles mosquito . Malaria ranks third among major infectious diseases in causing deaths after pneumococcal acute respiratory infections and tuberculosis and is a major cause of mortality and morbidity in India.[2] In the case of mosquito-borne transmission, sporozoites, the infective stage injected along with saliva into subcutaneous capillaries, enter hepatocytes. Inside the hepatocytes, each sporozoite begins a phase of asexual reproduction which results in the formation of a schizont containing thousands of merozoites.
The rupture of mature schizonts generates the liberation of merozoites into the bloodstream. The hepatic schizogony lasts approximately 5.5 days in P. falciparum. In case of P.vivax infections, a percentage of parasites may remain dormant in hepatocytes as hypnozoites for several months up to 5 years. The hepatic schizogony is asymptomatic, as only a few number of liver cells is infected. Merozoites reach and invade red cells rapidly to start a process of asexual multiplication (erythrocytic schizogony).Cytokines lead to the myriad manifestations.[3] Severe malaria as described by WHO , is manifested as clinically, impaired consciousness or unrousable coma, prostration, i.e. generalized weakness so that the patient is unable walk or sit up without assistance, failure to feed, multiple convulsions (more than two episodes in 24 h), deep breathing, respiratory distress (acidotic breathing), circulatory collapse or shock, with systolic blood pressure < 70 mmHg in adults, clinical jaundice plus evidence of other vital organ dysfunction, haemoglobinuria, abnormal spontaneous bleeding and pulmonary oedema (radiological).[4] Laboratory findings include hypoglycaemia (blood glucose < 40 mg/dl), metabolic acidosis (plasma bicarbonate < 15 mmol/l), severe normocytic anaemia (Hb< 5 g/dl, packed cell volume < 15%), haemoglobinuria, jaundice (serum bilirubin >3mg/dl) hyperparasitaemia (> 2% or 100,000/μl in low intensity transmission areas or > 5% or 250,000/μl in areas of high stable malaria transmission intensity), hyperlactataemia (lactate > 5 mmol/l) and renal impairment (serum creatinine > 3mg/dl)[4] Recent reports of malaria cases in India showed that 8.8% of the total malaria cases and 4.1% of the total falciparum cases were reported from Western India, hence due to the widespread prevalence of malaria in this region, this study was planned here.[5] Recently, it was shown that P. vivax was responsible for many of the complications traditionally associated with
P. falciparum and mixed malarias. Hence physicians need to know that this type of malaria may not be necessarily benign.[6] Also, literature on mixed malarias is scanty.[7] The aim of this study was to assess the prevalence of different types of malaria and complications as a whole and of each type. This would help primary care physicians to understand the importance of detecting complications early and institute treatment according to the type and presence of complications. Irrational use of anti malarials would therefore be curbed and each complication dealt with accordingly.
METHODS This cross sectional, prospective and observational study was conducted in a tertiary care centre in western India over a period of two years. Institutional ethical clearance was obtained from the college ethical committee and individual consent taken from each patient. For this study, a hundred patients with age more than 14 years, who were admitted to intensive care units and general medicine wards with a diagnosis of malaria done by a standard peripheral blood smear examination to demonstrate the parasite, were selected. Patients who had a history of systemic illnesses such as hypertension, diabetes, chronic kidney disease, tuberculosis, nephritis, chronic liver disease, acute or chronic viral hepatitis, and those on medications which would affect liver and renal function tests, were excluded.
Patients who had pneumonia, urinary tract infection and leptospirosis were also excluded. Patients who fulfilled inclusion criteria were asked about complaints such as fever, chills, malaise, bodyache, jaundice, oliguria, hematuria, swelling over body, breathlessness, bleeding tendencies, altered sensorium, convulsions etc. On admission, vital parameters like temperature, pulse rate, blood pressure and respiratory rate were recorded. Signs such as pallor, icterus, edema, bleeding were noted. Systemic examination was done to assess respiratory, cardiovascular, nervous and gastrointestinal systems. Investigations like peripheral smears, complete blood counts, platelet count, urea and creatinine, bilirubin, liver enzymes and blood sugar were done. Arterial blood gas (ABG) estimation was done in patients complaining of dyspnea. Malaria was diagnosed by gold standard method of examination of peripheral blood smear (PBS) and demonstration of asexual form of plasmodium. The PBS was made from a finger prick, thin, fixed with methanol and stained with diluted Giemsa with buffered water at pH of 7.2. It was seen under an oil immersion lens and a minimum of hundred fields were examined before declaring the PBS negative. Parasite density index was calculated for all patients being included. Severe complicated malaria was diagnosed according to the guidelines of World Health Organization.
Cerebral malaria was diagnosed when a patient had unarousable coma using Glasgow Coma Scale with exclusion of other etiologies with multiple convulsions - more than two episodes in 24 hours.[4] Renal failure, jaundice, hypoglycaemia and severe anemia were diagnosed when serum creatinine was more than 3.0 mg/dl, serum bilirubin was more than 3.0 mg/dl, random blood glucose was less than 40 mg/ dl and hemoglobin was less than 5.0 gm/dl, respectively.[4] Metabolic acidosis was labelled if plasma bicarbonate was less than 15 mmol/l. Circulatory collapse (hypotension) was defined as systolic blood pressure (BP) 30/min) with or without pulmonary edema (radiological).[4] All patients with cerebral malaria, renal impairment, ARDS were treated in the intensive care unit. Rest were managed in wards.
All forms of severe malaria and falciparum and mixed infections were treated by artesunate combination therapy and appropriate supportive therapy.[4] Statistical analysis : Data collected was systematically tabulated and analysis was done using standard statistical software SPSS version 15.0. For categorical data, chi- square test was used P< 0.05 was considered as a statistical significance at 95% confidence intervals. Multi variate analysis was done using multilinear regression analysis for various complications of falciparum malaria and outcome.
RESULTS Out of the total patients of malaria,50 % had P.vivax,35% had P.falciparum and 15% had mixed infection. Maximum patients, that is 33%, belonged to age group 21-30 years. 67% of the patients were males and 33% were females. Fever was present in 100% of patients. It was of intermittent type coming every day at an interval of 24 hours in 50% patients out of which 35 were of P. falciparum and 15 were of mixed infection. The fever was intermittent, coming every third day in 50 % patients, all of whom had P. vivax infection. Malaise and bodyache were observed in all patients. Vomiting was seen in 10 patients (10%) and malaena was reported by 5 patients (5%). 30 patients (30%) reported yellowish discolouration of sclera while 15(15%) reported decreased urine output. 13 patients (13%) presented with multiple convulsion (cerebral malaria) and 11 patients (11%) had tachypnea of more than 30 cycles per minute with saturation of oxygen below 92%. On ABG, all these patients showed acute respiratory distress syndrome (ARDS). Out of the total 100 study subjects, 53% had complicated malaria while 47% were uncomplicated cases.
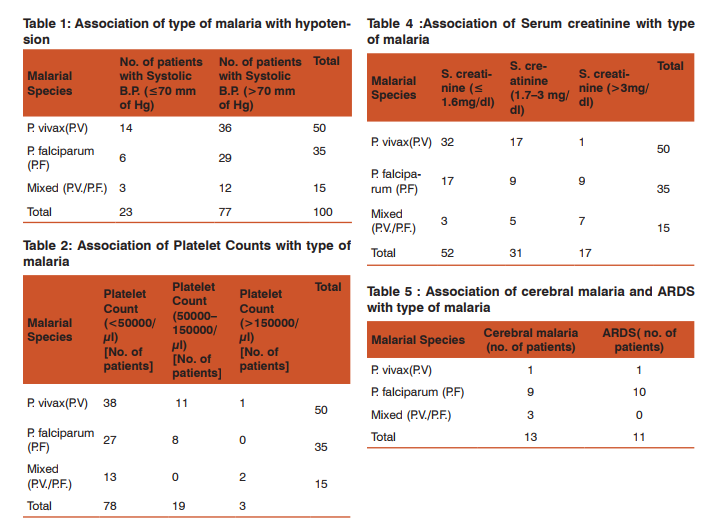
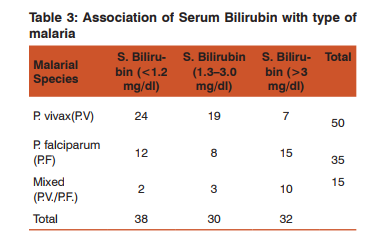
Of the total patients, 23% had hypotension with a systolic blood pressure (BP) of less than 70mmHg, 78% had severe thrombocytopenia, 10% had severe anemia (hemoglobin < 5g/dl), 32% had significant jaundice (bilirubin> 3mg/dl), and 48% had renal impairment out of which 17 patients had creatinine >3mg/dl. Cerebral malaria was seen in 13% patients, ARDS in 11% patients (radiologically and on arterial blood gases) while 5 patients (5%) had metabolic acidosis (bicarbonate levels < 15mmol/l). 14 patients (14%) had a prothrombin time of >15 seconds (coagulopathy). Out of 50 patients of P. vivax malaria, 14(28%) had hypotension (systolic blood pressure below 80mmHg. Out of 35 patients of P. falciparum, 6 (17.14%) and out of 15 patients of mixed malaria, 3(20%) had hypotension (Table 1). 38 patients (76%) of P. vivax patients had severe thrombocytopenia while 27 (77.14%) patients of P. falciparum and 13 (86.66%) of mixed malaria patients had it (Table 2). 7 patients of P. vivax( 14%) had jaundice with serum bilirubin more than 3mg/dl while 15 (42.86%) of P. falciparum and 10(66.67%) of mixed malarial infection had it (Table 3).
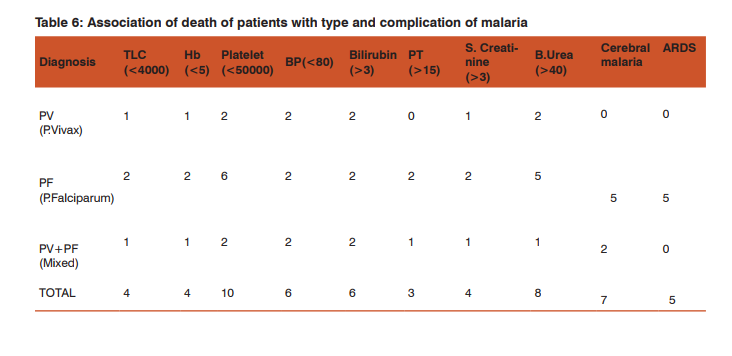
Only 1 patient (2%) of P. vivax malaria had serum creatinine of more than 3mg/dl while 9 (25.7%) patients of P. falciparum and 7 (46.67%) of mixed infection had this level of renal impairment (Table 4). 1 patient (2%) of P. vivax malaria had severe anemia with hemoglobin(Hb) below 5gm/dl while 8 (22.86%) of P. falciparum and 1 (6.67%) of mixed infection had this low Hb. Out of 50 patients of P. vivax, 4% had prolonged prothrombin time ,while in falciparum, the figure was 0.7% and in mixed infection, the value was 0.6%. Out of 13 patients having cerebral malaria, 9 had falciparum, 3 had mixed infection and 1 had vivax infection. In the 11 patients of ARDS, 10 had falciparum and 1 had vivax infection. Among 5 patients of metabolic acidosis, 3 were of falciparum and 2 of mixed infection (Table 6). Out of 17 patients having severe renal impairment, 8 underwent hemodialysis. All patients of ARDS ,severe renal impairment and cerebral malaria were treated in the ICU. Hence, severe thrombocytopenia and jaundice were the two most common complications found in this study. P. vivax accounted for more number of patients with hypotension than the other types of malaria but this difference was not found to be statistically significant.
Also, number of patients of severe thrombocytopenia in vivax and other malarias were comparable. The rest of the complications were predictably more in falciparum and mixed infections. Interestingly, cerebral malaria and ARDS were seen in one vivax patient each. In this study, there were 90% survivors and 10% of patients succumbed. Hence, the case fatality rate was 10%. Out of 90 survivors, 43 patients (47.7%) had some form of complicated malaria, whereas all patients who succumbed (10) had complicated malaria. All 10 patients (100%) had platelet counts of 3mg/dl in 4 patients (40%). Hypotension and jaundice was present in 3 patients (30%). Severe anemia was observed in 4 patients out of 10(40%). 7 patients had cerebral malaria (70%) and 5 had ARDS(5%) (Table 6). The case fatality rate for cerebral malaria was 54% and of ARDS was 45% respectively. All forms of severe malaria were treated by artesunate combination therapy as recommended by latest guidelines of WHO.[4]
DISCUSSION The prevalence of complicated malaria has significantly increased in the last decade. Western India has been hypoendemic for malaria with certain pockets of P.falciparum.[5] With a spurt in developmental activity in this area and increased irrigation, there has been a change in clinical profile of malaria in this region. Malaria caused approximately 929 000-1 685 000 deaths worldwide in 2010.[2] Resistance of mosquitoes to insecticide and chloroquine resistance has lead to an increase in complicated malaria. Over the last many years, jaundice and acute renal failure have been increasingly noticed in patients with malaria. Severe malaria has been defined by WHO as presence of one or more pernicious syndromes in a patient with asexual forms of plasmodium species in his blood.[4] According to annual report of Government of India, 38.6% of all cases from different parts of the country were due to P.falciparum. [8] . In this study,35% of patients were positive for P.falciparum. In a study by Kochar DK et al, 58.2% of patients were positive for P.falciparum.[6] In this study,67% of the patients were males and 33% were females and majority of patients (74%) were between ages of 21-50 years. According to a study by Das et al, burden of malaria is higher in males than females. [9]
Over the last 10 years, it has been noted that clinical profile of malaria has undergone a significant change in India. The present study revealed that severe thrombocytopenia was present in 78%, jaundice and hepatic dysfunction in 62%, renal failure in 48% , hypotension in 18%, cerebral malaria in 13% ,ARDS in 11%, severe anemia in 10% and metabolic acidosis in 5%. P. vivax accounted for more patients with hypotension than the other types of malaria. Also, number of patients of severe thrombocytopenia in vivax and other malarias were comparable. The rest of the complications were predictably more in falciparum and mixed infections. According to Kochar DK et al, cerebral malaria (25.75%), hepatic involvement (11.47%), spontaneous bleeding (9.58%), hemoglobinuria (7.89%), severe anemia (5.83%), algid malaria (5.26%), ARDS (3%) and renal failure (2.07%) were the important manifestations observed in P. falciparum infections.
The overall mortality was 11.09%.[10] In another study by Kochar DK et al, complications observed in P. Vivax malaria were hepatic dysfunction and jaundice in 23 (57.5%) patients, renal failure in 18 (45%) patients, severe anemia in 13 (32.5%) patients, cerebral malaria in 5 patients (12.5%), acute respiratory distress syndrome in 4 patients (10%), shock in 3 patients (7.5%), and hypoglycemia in 1 (2.5%) patient. Thrombocytopenia was observed in 5 (12.5%) patients, and multi-organ dysfunction was detected in 19 (47.5%) patients. [6] In another study done by Naha K et al, anaemia was seen in 65 (30.5%), leucopenia in 38 (17.8%) and thrombocytopenia in 184 (86.4%) patients of P. vivax infection. Hepatic dysfunction was noted in 40% and hypoalbuminemia was observed in 157 (73.6%) cases.
Elevated serum creatinine was noted in in 59 (27.5%) patients. Overall, 107 (50.2%) patients fulfilled WHO criteria for severe malaria.[11] In this study, many patients with mixed infections presented with complications such as hypotension, thrombocytopenia, jaundice ,renal impairment and cerebral malaria. But in a study by Luxemberger C et al, severity of P. falciparum is attenuated by concomitant P. vivax infection.[12] Hence, P.vivax is no longer the benign infection it was and a high index of suspicion for its complications has to be kept by primary care physicians so that appropriate referrals can be made. Falciparum and mixed malarias are the most dangerous infections and complications involving all major organs occur in a vast number of patients and hence these should be picked up fast and managed without delay.
CONCLUSION The complications seen in this study were mainly severe thrombocytopenia , hypotension, jaundice, renal impairment, cerebral malaria, ARDS, severe anemia and metabolic acidosis; severe thrombocytopenia and jaundice being the commonest, and. P. vivax accounted for more patients with hypotension than the other types of malaria. Predictably, other complications were more in the falciparum group. But the numbers were many in the vivax group also, although not statistically significant. Mixed malaria accounted for severe complications as well.
ACKNOWLEDGEMENT The authors acknowledge the help and support received from the head of the institute and hospital superintendent, as well as all the nursing and support staff. Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
Source of Funding : Self
Conflict of interest: no conflict of interest amongst authors
References:
1. Park JE, Park K. Textbook of Preventive and Social Medicine,16th Edition, 1998;188-201.
2. Murray CJL, Rosenfeld LC, Lim SS et al. Global malaria mortality between 1980 and 2010: a systematic analysis. The Lancet 2012;379(9814):413-431.
3. Gilles HM. The malaria parasites. In: Warrell DA, Gilles HM, editors. Essential malariology. Arnold; 1993. pp. 12–34.
4. WHO. Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg 2000;94(Suppl 1): S1-90.
5. Centre for disease control and prevention, Malaria Facts 2004
6. Kochar DK, Das A, Kochar SK,Saxena V, Sirohi P, Garg S et al. Severe P.Vivax malaria: A report on serial cases from Bikaner in Northwestern India. Am J Trop Med Hyg 2009;80(2):194-198.
7. Joseph V, Varma M, Vidhyasagar S, Matthew A. Comparison of the Clinical Profile and Complications of Mixed Malarial Infections of Plasmodium Falciparum and Plasmodium Vivax versus Plasmodium Falciparum Mono-infection. Sultan Qaboos Univ Med J. 2011 Aug; 11(3): 377–382.
8. Government of India, Annual Report 1995-96.DGHS,New Delhi
9. Das NG, Baruah I, Kamal S, Sarkar PK, Das SC, Santhanam K. An epidemiological and entomological investigation on malaria outbreak at Tamalpur PHC,Assam. Indian J Malariol 1997;34:164-170.
10. Kochar DK, Kumawat BL,Karan S, Kochar SK, Aggarwal RP et al. Severe and complicated malaria in Bikaner, Rajasthan,Western India. Southeast Asian J Trop Med Public Health 1997;28(2):259-267.
11. Naha K, Dasari S, Prabhu M. Spectrum of complications associated with P. Vivax infection in a tertiary hospital in SouthWestern India. Asian Pacific Journal of Tropical Medicine 2012; 5(1): 79-82.
12. Luxemburger C, van Vugt M, Jonathan S, McGready R, Looareesuwan S, White NJ, Nosten F. Treatment of vivax malaria on the western border of Thailand. Trans R Soc Trop Med Hyg. 1999;93(4):433-8.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License