IJCRR - 13(7), April, 2021
Pages: 118-123
Date of Publication: 12-Apr-2021
Print Article
Download XML Download PDF
Bio-conversion of Lignin Extracted from Sugarcane Bagasse and Coconut Husk to Vanillin by Bacillus sp.
Author: Harpreet Kaur, P V Pavithra, Sriya Das and M Kavitha
Category: Healthcare
Abstract:Introduction: Vanillin, chemically known as 4-hydroxy 3-hydroxybenzaldehyde is a white crystalline phenolic aldehyde and it is a commonly used flavouring agent. Vanillin is used as a flavouring agent in food and beverages as it contains an aromatic fla�vour. It also has applications in the pharmaceuticals and cosmetic industry. It is the second most expensive compound because growing vanilla seed pods is labour-intensive and it is estimated that only 0.2% of the global demand for vanillin is met by natural sources and the other relied source is a chemical synthesis that lacks efficacy. Hence an alternative approach is the production of vanillin by microbial bioconversion. Objective: The present study aims to produce vanillin from lignin extracted from agricultural waste by bio-conversion using Bacillus sp. Methods: Lignin was extracted by acid/alkali hydrolysis from sugarcane bagasse and coconut husk. It was further quantified and confirmed by Fourier-Transform Infrared Spectroscopy analysis. The lignin thus obtained was used to produce vanillin by microbial conversion using Bacillus sp. It was quantified and confirmed by FTIR. Results: The yield of vanillin obtained was 0.7 mg/ml by spectrophotometric analysis. Conclusion: The bioconversion of lignin to vanillin is tremendously advantageous as it is efficient, cost-effective and ecologi�cally safe.
Keywords: Vanillin, Lignin, Sugarcane bagasse, Coconut husk, Microbial bioconversion
Full Text:
Introduction
Vanillin chemically known as 4-hydroxy 3-hydroxybenzaldehyde is a white crystalline phenolic aldehyde and it is a commonest flavouring and perfuming agent. It is naturally extracted from Vanilla planifolia which is an orchid, also known as a vanilla bean.1 Its fragrance and taste are from its fruit, vanilla pod, by an enzymatic process called curing post-harvest. After the curing process, only 2% of the vanillin is extracted. It is also a labour-intensive process leading to its high cost.2 Due to the shortage and high cost, synthetic vanillin production was researched and adopted. The methods of commercial production of vanillin were first started by using natural and readily available, eugenol also known as clove oil (4-allyl-2-methoxyphenyl).3 Later other methods of the artificial production of vanillin were followed using lignin or guaiacol. 85-90% of the production depends on guaiacol, which is a phenolic ether also known as 1-Hydroxy-2-methoxybenzene and only 10-15% is from lignin. These synthetic methods produce “naturally similar” vanillin but not natural vanillin. Lignin is the second most abundant natural polymer with cellulose being number one, making up to 10–25% of lignocellulosic biomass.4,5
The natural vanillin extract meets the global vanillin demand by around only 1%.1 It is used as a flavouring agent mainly in ice creams and chocolate manufacturing (accounting for 75%), followed by the pharmaceutical industry to mask the undesirable flavour, and food and beverages.6 Vanillin is also useful for visualization of alcohols, ketones and aldehydes separated using thin layer chromatography.7 Vanillin-HCl staining is used for tannins histochemical testing.8 Vanillin is also used as a food preservative as it contains anti-bacterial and anti-oxidant activities.9 Due to its high demand, in the recent past researcher are exploring alternative methods for the production of vanillin. To meet the expanding demand for vanillin, biotechnological approaches such as bioconversion of ferulic acid, isoeugenol, eugenol, lignin with the help of bacteria, fungi and genetically modified microorganisms are being researched to explore its full potential.1,10,11 Microbes such as white-rot fungi, bacteria such Bacillus sp. are used to degrade the complex lignin polymer to vanillin, ferulic acid and vanillic acid.12 The present study is aimed to explore the potential of Bacillus sp. in the bioconversion of lignin extracted from coconut husk and sugarcane bagasse to vanillin.
Materials and Methods
Materials
Vanillin and catechin were procured from Sigma. Mineral Salts Medium (MSM) and other culture media ingredients were from Hi-Media, Mumbai. All other chemicals used in the study were of analytical grade obtained locally.
Growth conditions for Bacillus sp.
The Bacillus sp. procured from ATCC and available for regular work in the Microbiology laboratory was used in the study. It was cultured in Minimal salts medium (MSM) at 37°C for 24 hours. Stock cultures were maintained at 4°Cfor for further use. Gram’s staining was done to confirm its characteristic morphology and endospores.
Extraction of lignin from sugarcane bagasse and coconut husk
Sugarcane bagasse and coconut husk were collected from local juice shop and farmland in Vellore respectively. (Figure 1-2) Lignin from sugarcane bagasse was extracted by acid hydrolysis. It was shredded into smaller pieces, mixed with formic acid and acetic acid mixture (70:30) and boiled for 2 hours. The residues were filtered, treated with peroxyformic/ peroxyacetic acid which was prepared using 35% H2O2 and boiled at 80oC for 2 hours. The lignin present in the residues was precipitated with distilled water.13
Lignin from coconut husk was extracted by alkali hydrolysis. Coconut husk was cut into smaller pieces, oven-dried and processed with 1M aqueous NaOH solution at 100oC for 1 h. It was cooled and then filtered. The filtered residue was precipitated with 20% phosphoric acid and kept overnight.14 The lignin precipitate was separated using filter paper. The separated lignin was placed in a Petri plate and dried in an oven at 55oC for 48 h till the lignin was dried completely.
Characterization of Lignin by Fourier-Transform Infrared Spectroscopy analysis
The extracted lignin was structurally characterized using Fourier transform infrared spectroscopy (Shimadzu). The scan was performed in the range of 500-4000 cm-1.15
Bioconversion of lignin to vanillin
The bioconversion of extracted lignin to vanillin was carried out in MSM media supplemented with 0.5% and 1% lignin using Bacillus sp. The culture media were prepared, sterilised and inoculated with 1% inoculum of Bacillus sp. The culture flasks were incubated at 37oC for 48 h under shaking condition. After incubation, the culture fluid was centrifuged at 8000 rpm for 10 min at 4oC to obtain the supernatant containing vanillin for further analysis.
Vanillin assay
Thiobarbituric acid assay
Thiobarbituric acid reacts with vanillin to form a yellow coloured product with maximum absorbance at 434 nm which can be measured using a spectrophotometer. To 1 ml of the culture supernatant, 5 ml of 24% HCl and 2 ml of 2% thiobarbituric acid were added and mixed. The test tubes were incubated at 55oC for 10 min in a water bath followed by 20 min at room temperature. The absorbance was measured at 434 nm and compared with vanillin standard to determine the concentration of vanillin present in the supernatant.1
Catechin assay
Aromatic aldehydes when reacts with meta substituted flavanols gives a red adduct with maximum absorbance at 500 nm. 8% Hydrochloric acid (HCl) was prepared in methanol. 0.3% catechin was prepared and stored in a dark bottle at 4oC. To 1 ml of the culture supernatant, 5ml HCl reagent and 5 ml catechin were added and incubated at 50oC in the water bath for 20 min to form a red coloured adduct. The absorbance was measured at 500 nm and compared with vanillin standard to determine the concentration of vanillin present in the supernatant.16
Characterization of vanillin by High-Performance Liquid Chromatography (HPLC) analysis
The vanillin was analysed using WATERS High-Performance Liquid Chromatography. The mobile phase was acetonitrile and 0.2% acetic acid in the ratio of 60:40. 20 microliter of vanillin was injected into the column and the flow rate was adjusted 1ml/minute and the graph was obtained at 280nm and compared with vanillin standard.1,16
Results and Discussion
Morphology of Bacillus sp.
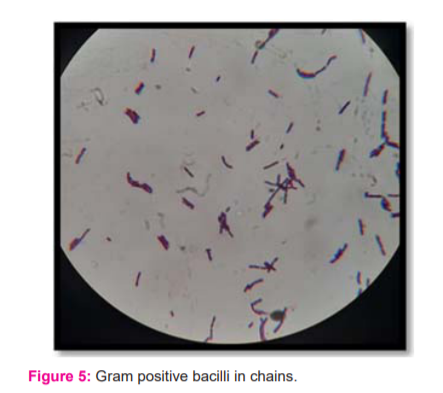
Bacillus sp. presented with grey-white, round, opaque and medium-sized colonies on both nutrient agar and Lignin-MSM plates (Figure 3-4). Gram staining revealed the presence of Gram-positive bacilli, arranged in chains with endospores (Figure 5).
Extraction of lignin from sugarcane bagasse and coconut husk
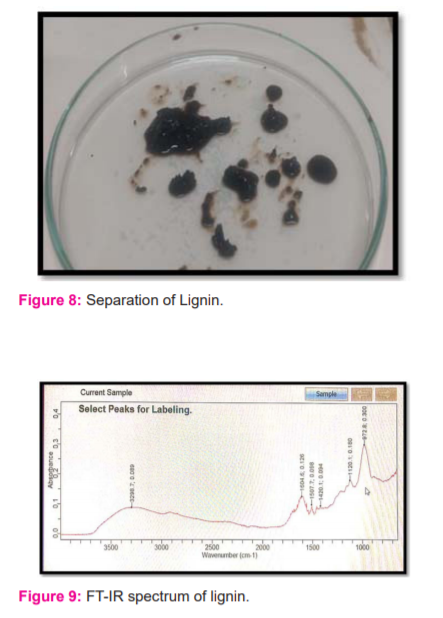
In both acid hydrolysis of sugarcane bagasse and alkali hydrolysis of coconut husk, the dark brown semisolid precipitate was obtained on Whatman No.1 filter paper. (Figures 6-8) This precipitate was dried and used in further study. The yield of lignin was 6% from sugarcane bagasse and 7% from coconut husk.
Characterization of lignin by FT-IR analysis
The FTIR analysis of the given sample (Figure 9) was carried out and compared with the reference wave numbers of lignin reported in the literature.15 It could be observed that the wavenumbers fairly agreed with the values reported in the literature proving the presence of lignin in the extract (Table 1). In FT-IR analysis the stretching patterns indicate the presence of compounds like phenols, hydroxides, methyl groups, which are present in lignin. The bands in the region of 1600 and 1500 cm-1 indicate the presence of aromatic compounds such as phenol (Figure 9).
Bioconversion of lignin to vanillin
After the bioconversion reaction, the light brown supernatant obtained (Figure 10) was subjected to vanillin assay.
Thiobarbituric acid assay
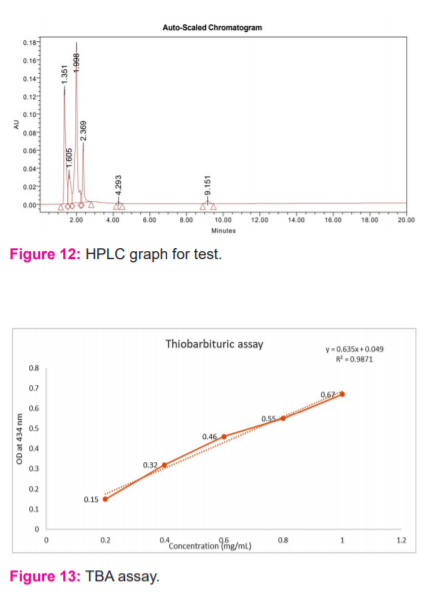
Upon the addition of thiobarbituric acid to the tests and standards, the yellow coloured product was observed in the test sample. The absorbance was read at 434 nm and recorded and a standard graph was plotted. (Figure 13) The standard curve was calibrated from 0.2- 1 mg/ml. The regression equation obtained was y= 0.635x + 0.049. The r2 value obtained was 0.9871. From the graph, it could be interpreted that the vanillin present in the broth is 0.7 mg/ml.
Catechin assay
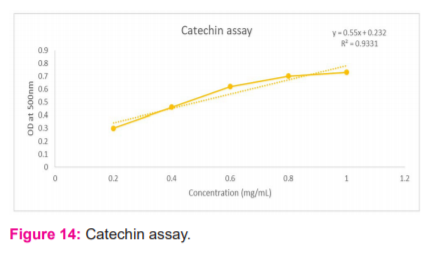
The tests and standards reacted with catechin and resulted in the formation of a light red coloured product. The absorbance was recorded at 500nm. The standard curve was calibrated from 0.2- 1 mg/mL. The regression equation obtained was y= 0.55x + 0.232. The r2 value obtained was 0.93. From the graph, it is interpreted that the vanillin present in the sample is estimated to be 0.65 mg/mL (Figure 14).
Characterization of vanillin by HPLC analysis
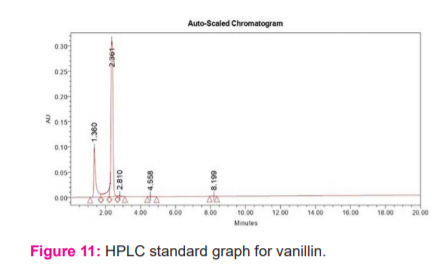
The extracted vanillin was analysed using WATERS High-performance liquid chromatography. As seen in the HPLC graph (Figure 11), the retention time of vanillin was 2.361. Few other smaller peaks were observed due to the tendency of vanillin to rapidly oxidize to vanillic acid and structural similarity between them. The formation of vanillic acid hence may have interfered in the specific detection of vanillin by HPLC. The other reason for various peaks might be a small number of impurities present in solvent or standard vanillin. Observing one of the peak retention time 2.369 of the test sample (Figure 12), we could conclude that vanillin was present in the sample. Most of the reports on lignocellulosic biomass have been on its lignification, structural properties and degradation.17,18 The setbacks in the commercial production of vanillin lies in its chemical reactivity and it is rapidly converted to vanillic acid. 19 Current research on the elucidation of the biosynthetic pathway of vanillin in microorganisms opened up new opportunities in the biotechnological production of vanillin.20
Conclusion
This study demonstrated the capability of Bacillus sp. to adapt in the lignin- MSM media and the bioconversion of lignin to more valuable product vanillin. The lignin was retrieved by acidic and alkaline methods and H2O2 accelerated the process. Lignocellulosic biomass has been explored more on its lignification, structural properties and degradation. The shortcoming in the biotechnological production of vanillin includes its chemical reactivity. Vanillin gets rapidly converted to vanillic acid. To make this process industrially and economically viable it is important to inhibit the conversion of vanillin, make it more stable and develop strategies to recover it more efficiently. The increasing knowledge regarding enzymes that are involved in pathways for the biosynthesis and metabolism, identification and characterization of the corresponding genes, offers new opportunities for metabolic engineering and the construction of genetically engineered production strains to advance the production.
Acknowledgement
The authors thank the Vellore Institute of Technology for providing all necessary equipment and chemicals to carry out this project work.
Conflicts of Interest
NIL
Funding
NIL
Authors Contribution
All the authors were involved in the conception and design of the work, experimental works, data collection, data analysis and drafting of the article. The corresponding author revised and approved the final version of the manuscript.

References:
-
Rana R, Mathur A, Jain CK, Sharma SK, Mathur G. Microbial production of vanillin. Int J Biotechnol Bioengi Res 2013;4 (3):227-234.
-
Dignum MJ, Kerler J, Verpoorte R. Vanilla production: technological, chemical, and biosynthetic aspects. Food Rev Int 2001;17(2):119-120.
-
Kaur B, Chakraborty D. Biotechnological and molecular approaches for vanillin production: a review. Appl Biochem Biotechnol 2013;169(4):1353-1372.
-
Pothiraj C, Kanmani P, Balaji P. Bioconversion of lignocellulose materials. Mycobiology 2006;34(4):159-165.
-
Pearl IA. Vanillin from lignin materials. J Am Chem Soc 1942;64(6):1429-1431.
-
Vanillin: Physiochemical Properties, Production and Uses. UKEssays November 2018.
-
Pirrung MC. Appendix 3: Recipes for TLC stains. The Synthetic Organic Chemist’s Companion. 2006:171-2.
-
Tai A, Sawano T, Yazama F, Ito H. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Biochimica et Biophysica Acta 2011;1810(2):170-177.
-
Gardner RO. Vanillin-hydrochloric acid as a histochemical test for tannin. Stain Technol 1975;50(5):315-317.
-
Karode B, Patil U, Jobanputra A. Biotransformation of low cost lignocellulosic substrates into vanillin by white rot fungus. Phanerochaete chrysosporium NCIM. Indian J Biotechnol 2013;12:281-283.
-
Muheim A, Lerch K. Towards a high-yield bioconversion of ferulic acid to vanillin. Appl Microbiol Biotechnol 1999;51(4):456-461.
-
Chen P, Yan L, Wu Z, Li S, Bai Z, Yan X, et al. A microbial transformation using Bacillus subtilis B7-S to produce natural vanillin from ferulic acid. Sci Rep 2016;6:20400.
-
Watkins D, Nuruddin M, Hosur M, Tcherbi-Narteh A, Jeelani S. Extraction and characterization of lignin from different biomass resources. J Materials Res Technol 2015;4(1):26-32.
-
Forss KG, Talka ET, Fremer KE, inventors; Forss Kaj G, Talka Esko T, Fremer Kaj Erik, assignee. Method for the isolation of vanillin from lignin in alkaline solutions. United States patent US 4,277,626. 1981 Jul 7.
-
Lu Y, Lu YC, Hu HQ, Xie FJ, Wei XY, Fan X. Structural characterization of lignin and its degradation products with spectroscopic methods. J Spectrosc 2017;2017: 8951658.
-
Graf N, Altenbuchner J. Genetic engineering of Pseudomonas putida KT2440 for rapid and high-yield production of vanillin from ferulic acid. Appl Microbiol Biotechnol 2014;98(1):137-149.
-
Kantharaj P, Boobalan B, Sooriamuthu S, Mani R. Lignocellulose degrading enzymes from fungi and their industrial applications. Int J Curr Res Rev 2017;9(21):1-7.
-
Glasser WG. About making lignin great again-some lessons from the past. Front Chem 2019;7:565.
-
Converti A, Aliakbarian B, Domínguez JM, Vázquez GB, Perego P. Microbial production of biovanillin. Braz J Microbiol 2010;41(3):519-530.
-
Zheng L, Zheng P, Sun Z, Bai Y, Wang J, Guo X. Production of vanillin from waste residue of rice bran oil by Aspergillus niger and Pycnoporus cinnabarinus. Bioreso Technol 2007;98(5):1115-1119.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License