IJCRR - 13(7), April, 2021
Pages: 80-85
Date of Publication: 12-Apr-2021
Print Article
Download XML Download PDF
Histomorphological Spectrum of Neoplastic Lesions of Kidney with a Brief Review of Literature
Author: Nalini Modepalli, Jyothi Anantharaj, Naveen Shivappa, Shwetha Basavaraj, Sakshi Barve
Category: Healthcare
Abstract:Introduction: Renal tumours are an important cause of morbidity worldwide. They include a diverse spectrum and the recent advances in the field of renal neoplasia has given a better understanding of the morphological and molecular characteristics leading to the new Vancouver classification and the new WHO classification of Renal tumours. Objective: To study the histomorphological features of various renal tumours. To classify them as per the new WHO classifica�tion of renal Tumors and its correlation with the clinical findings. Methods: A cross-sectional study of renal tumours diagnosed on the nephrectomy specimens received between January 2017 to December 2019 was carried out to study the various morphological types. Results: Of the 39 nephrectomy specimens received during the study period, 15 were neoplastic and 24cases were non-neo�plastic. Among neoplastic, 4 (26.6%) cases were benign and 11cases(73.3%) were malignant tumours, of which clear cell Renal Cell Carcinoma was the most common. Furhmann nuclear grade II was more frequently encountered in our study. The most common site was the upper pole of the kidney. The most common age of presentation was in the 7th decade. Conclusion: Reclassification of renal tumours based on their molecular, clinical and pathological features and the emergence of new entities has lead to a better understanding of renal neoplasms and also aids accurate diagnosis of these tumours leading to better management strategies.
Keywords: Kidney, Neoplasms, Morphology, Benign, Malignant
Full Text:
MAIN ARTICLE
Introduction
The kidneys are paired, bean-shaped organs located on either sides of the vertebral column in the retroperitoneal space. They are dynamic organs serving as the main osmoregulatory system (?uid –electrolyte balance) in humans. In addition to maintaining fluid homeostasis they also act as an endocrine organ (secrete hormones - prostaglandins, and regulating vitamin D metabolism) control Red blood cell production by secreting hormone erythropoeitin and regulate blood pressure through enzyme renin. 1[D1]
The renal parenchyma though subjected to repeated trauma/insults of the noxious environment, are the last to respond . They can be involved in various pathological processes, which can be diagnosed on biopsies and nephrectomy specimens. Various disease processes affect the kidneys, some resulting in permanent damage leading to surgical removal of the organ.
Nephrectomy is a common procedure in surgical practice and is of many types; partial, total and radical nephrectomy. A standard radical nephrectomy specimen consists of the entire kidney including the calyces, pelvis, variable length of ureter ,entire perirenal fatty tissue to the level of Gerota’s fascia and variable lengths of the major renal vessels at the hilus. The adrenal gland is usually removed en bloc with the kidney. Regional lymphadenectomy is not generally performed even with a radial nephrectomy. 2
A partial nephrectomy specimen may vary from a simple enucleation of the tumor to part of a kidney containing variable portions of calyceal or renal pelvic collecting system. The perirenal fat immediately overlying the resected portion of the kidney but not to a level of Gerota’s fascia is usually included. 2
Radical nephrectomy indicated in end stage renal disease, treatment of renovascular hypertension from non-correctable renal artery disease, or in severe unilateral parenchymal damage resulting from nephrosclerosis, pyelonephritis, Xanthogranulomatous pyelonephritis , vesicoureteric reflux, and congenital dysplasia. It is the treatment of choice in renal cell carcinomas. 3
Percutaneous image guided biopsy of renal masses is a safe and accurate procedure, being increasingly used to differentiate between benign and malignant entities to avoid unneccesary surgery. 4
In the last few years, there has been a growing interest on nephron-sparing surgery or partial nephrectomy to treat the selected cases of localized renal cell carcinoma (RCC) by open or laparoscopic approach.
Renal cancer is the 9th most common cancer in men accounting for 2.5% of total malignancies.WHO (2018). 5
The histological classification of RCCs is extremely important, considering the significant implications of the subtypes in the prognosis and treatment of these tumors. 6
In 2004 World Health Organization (WHO) published histological classification of renal tumors. 7 . In 2012, the International Society of Urological Pathology (ISUP) organized a conference in Vancouver, Canada which gave a new classification, referred to as “2012 ISUP Vancouver classification” (2012 ISUP).On the foundation of 2012 ISUP classification,2016 World Health Organization (WHO) renal tumour classification was published after considering new knowledge about pathology, epidemiology, and genetics. 3
Molecular and morphologic interrogation has driven a much-needed reexamination of renal cell carcinoma (RCC). Latest 2016 World Health Organization classification now recognizes 12 distinct RCC subtypes, as well as several other emerging/provisional RCC entities.( expanded from 4 subtypes in the 1997 Heidelberg classification to 12 recognized subtypes in 2016) 8
Important changes from previously classified renal tumour types , to the new 2016 WHO classification refers to subtypes that have been named on the basis of predominant cytoplasmic features (eg, clear cell and chromophobe renal cell carcinomas [RCCs]), architectural features (eg, papillary RCC), anatomic location of tumours (eg, collecting duct and renal medullary carcinomas), and correlation with a specific renal disease background (eg, acquired cystic disease– associated RCCs) as well as molecular alterations pathognomonic for RCC subtypes (eg, MiT family translocation carcinomas and succinate dehydrogenase [SDH]–deficient renal carcinomas) or familial predisposition syndromes (eg, hereditary leiomyomatosis and RCC [HLRCC] syndrome– associated RCC). 9
Emerging/provisional entities are TCEB1-mutated RCC/RCC with angioleiomyoma-like stroma/RCC with leiomyomatous stroma, RCC associated with ALK gene rearrangement, Thyroidlike follicular RCC and RCC in survivors of NB. 8
The Fuhrman system is the most frequently used grading system but has its limitations. The four-tiered WHO/ISUP grading system is recommended by the WHO . 9 However, this too has been validated as a prognostic indicator only for clear cell and papillary RCC and not for other morphotypes of renal cell neoplasms.
2016 WHO classification describes a number of new and emerging entities along with the previously recognized and also the provisional RCC types. This mandates the surgical pathologists to integrate clinical, radiologic, gross, and microscopic findings to successfully navigate challenging differential diagnoses in RCC classification. Unusual morphologic features ( clear cytoplasm, papillary architecture, and eosinophilic (oncocytic) cytoplasm) seen in various subtypes need to be kept in mind in our approach to various RCC’s.
Hence we intended to study the morphological subtypes of renal tumor using the new WHO classification.
Materials and methods
We conducted a cross sectional / observational study on all the nephrectomy specimens received in Central laboratory, Department of Pathology (Histopathology division) Rajarajeswari Medical College and Hospital, Bangalore, India from Jan 2017 to Dec 2019.
Inclusion criteria- Nephrectomy Specimens received in central lab, department of Pathology, Rajarajeswari Medical College and Hospital which on histopathological examination was diagnosed as Renal tumor listed under the WHO classification of tumors of kidney were included in this study.
Exclusion criteria- The non neoplastic lesions and metastatic tumors of kidney were excluded from the study.
Study Design - Specimens were received in 10% formalin. Essential clinical details including patient’s identification, age, sex, clinical data, investigations such as CT scan, USG, other relevant investigations were noted from the test request form. After proper fixation, weight and dimensions were recorded and the gross findings including examination of capsule, external surface and features on cut section were noted. Sections for histopathological examination from a lesion suspicious of tumor were taken from Tumor proper (multiple bits), Tumor with renal capsule, Tumor with Gerota’s fascia, hilar structures, Renal sinus, Renal pelvis , vessels , ureter and surgical margins. Lymph nodes(if any) and sections from adjacent kidney are taken to look for associated features in the adjacent renal parenchyma. Tissue was processed, embedded, sections were cut and stained by Haematoxylin and Eosin stain and submitted for histopathological examination. Microscopic features were noted and final reports were signed out as per the CAP protocol.
RESULTS
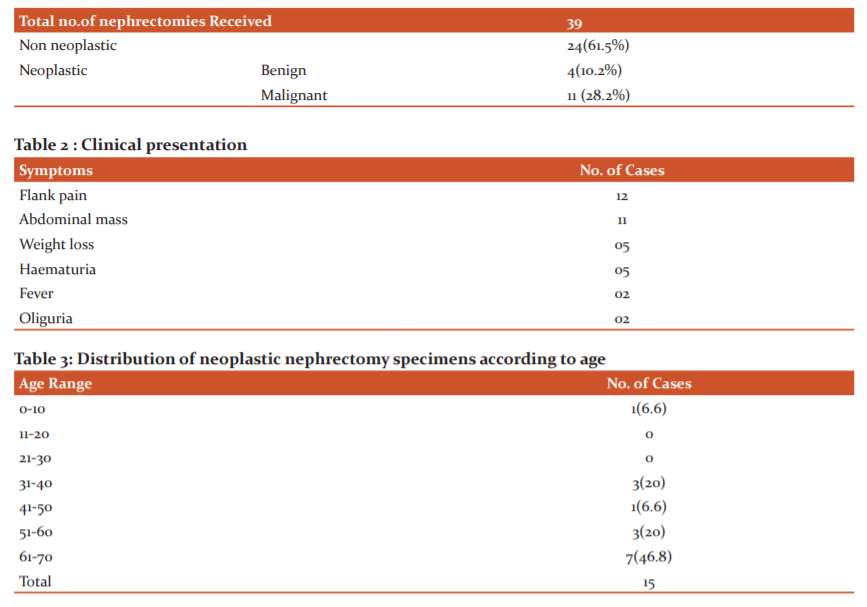
A total of 39 nephrectomy specimens were received during the study period, out of which 15 cases(38.4%) were neoplastic and 24cases (61.6%)were non neoplastic. Among neoplastic, 4 (26.6%) cases were benign and 11cases(73.3%) were malignant tumors.
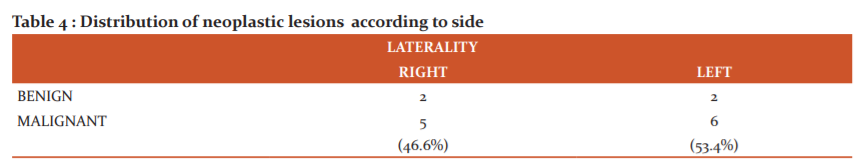
Flank pain (12 cases) was the most common clinical presentation in Renal tumors followed by abdominal mass(11 cases). The highest percentage of patients belong to 61-70years age group (46.6%) with female preponderance. Tumors were more common on the left than the right side with a predilection to the upper pole (40%). Clear cell renal cell carcinoma was more common malignant tumor and angiomyolipoma was the common one among the benign ones. Of the 10 Renal cell carcinomas, Fuhrman nuclear grading 2 was marginally more common(4 cases).
Discussion
Kidney are by far very resilient organs capable of withstanding the repeated stimuli by various disease processes. Once a permanent irreversible damage sets in, nephrectomy is indicated.
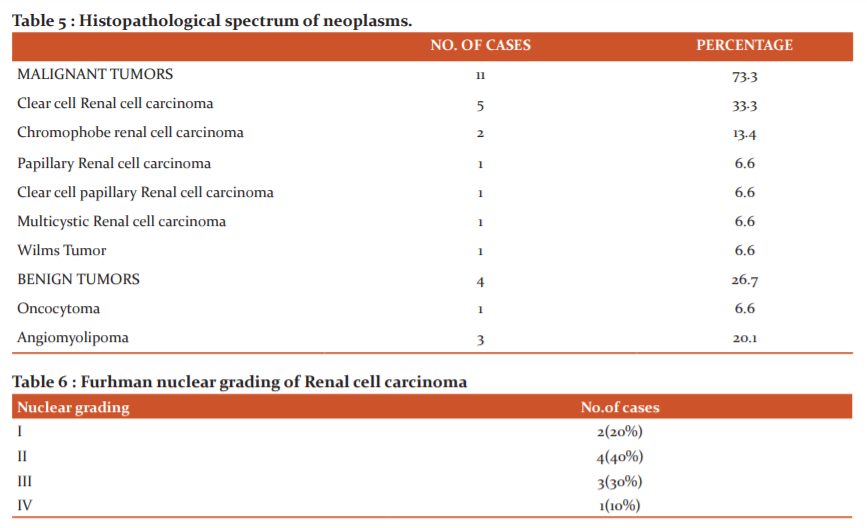
In our 2 year study , 15 primary renal neoplastic lesions were identified among the 39 nephrectomies received in that period. Malignant tumors accounted for 73.3% cases which was concurrent with the studies done by Vinay KS et al 10 , Reddy et al 11 , Basir et al 12 and Gafoor A et al 13.
Mean age in present study is 42.7yrs with majority of patients in the age group of 60- 70yrs , youngest being 1year old with Wilms tumour. These findings were consistent with study conducted by Narang et al. 14
There was female preponderance in our study with with male:female ratio of 1:1.2.This finding was in correlation with studies by Aiman et al 3 and Shifa et al 15 but most of the other studies showed a male preponderance.
Left kidney showed a higher incidence of neoplastic lesions in our study as was in the studies by Chaitra.B et al 16 and Swarnalata Ajmera et al. 17
Most of the patients presented with flank pain(12 cases) and abdominal mass(11 cases) , a finding similar to study conducted by Bharti et al. 18
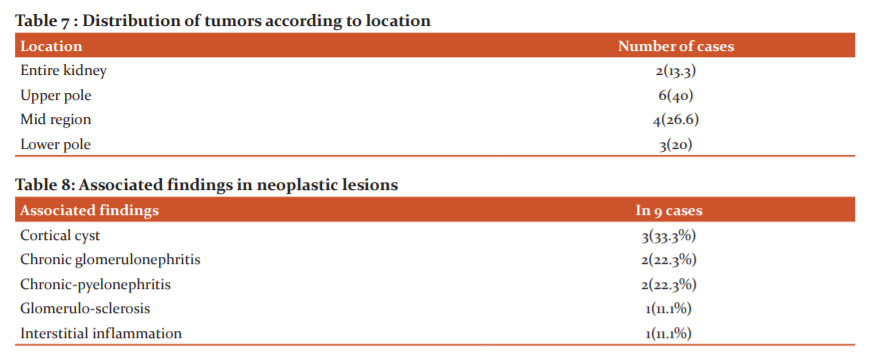
Grossly, most of tumors were situated in upper pole(40%) in our study comparable to Popat et al. 19
The most common malignant tumor was RCC (46.6%) , among which clear cell RCC was the most common histologic variant, consistent with the available literature. This was followed by chromophobe type RCC (2/11malignant tumors) followed by one case each of Papillary RCC, Clear cell papillary RCC (with low grade clear cells in tubules and papillae), Multicystic RCC.
Clear cell papillary renal cell carcinoma (CCP-RCC) is a recently proposed, distinctive, uncommon and indolent renal epithelial neoplasm, thought to arise in end-stage renal disease. 20 They are often small, well- circumscribed, and encapsulated, accounting for 1-4% of all resected renal tumours. These tumors have immunophenotype and molecular profiles distinct from those of clear cell and papillary RCCs. 21
CCP-RCC is composed of bland clear epithelial cells arranged in tubules and papillae, with linear nuclear alignment away from basement membrane and low Fuhrman nuclear grade. Most tumours are WHO I International Society of Urological Pathology (ISUP) grade 1 or 2. 21 Clear cell RCC rarely has papillary architecture while papillary RCC only rarely contains clear cells. Primary RCCs with both papillary architecture and cells with clear cytoplasm are diagnostically challenging for pathologists. Clues to the diagnosis of CCP-RCC include unique subnuclear cytoplasmic clearing, low nuclear grade, prominent smooth muscle stroma, and in some cases an end-stage background kidney. The morphology and typical IHC pattern of positive CK7 and CAIX but negative CD10 and AMACR should lead to accurate classification. Absence of 3p loss or trisomy of chromosomes 7 and 17 support the above diagnosis. Despite these tools, some RCCs with papillary architecture and clear cells do not fit into any of the described entities and currently remain unclassified. 22
One case of Clear cell RCC showed sarcomatoid features with 50-60% of cells arranged in solid sheets of spindle cells with high pleomorphism and high nuclear grade (Fuhrman grade 4) .
Sarcomatoid RCC (sRCC) is no longer considered a separate tumor type as it can occur with all histologic subtypes. 23 It is now recognized as a high-grade undifferentiated/ dedifferentiated component of RCC, characterized by both epithelial and mesenchymal differentiation. These components share a common cell of origin as suggested by their genomic features. 24 The sarcomatoid pattern may result from activation of a distinct sarcomatoid stem cell within the tumor. The average incidence is 8% among all RCCs. 24
These tumors contain features similar to sarcomas, with spindle-like cells, high cellularity, and cellular atypia. These rare tumors show variable amounts of carcinoma elements and barely any classic RCC areas. Hence IHC using epithelial and mesenchymal is mandatory to distinguish sarcomas from sRCC - sarcomatoid areas express cytokeratin AE1/AE3 and are negative for mesenchymal markers, such as desmin and actin. 23
These tumors are aggressive, progress rapidly and carry poor prognosis, irrespective of the underlying RCC subtype. These patients have a reported median survival time of 4-9 months after diagnosis. Major prognostic factors include proportion of sarcomatoid components, pathological stage, tumor necrosis, tumor size and genetic factors. Distant metastases are common in the lungs and bones. 24
One case of Wilms tumor in a one year old was reported in the study.
In present study Furhman grade –II was seen more commonly among the Renal cell carcinomas, which was comparable with results of the study by Aiman et al. 3
Among the 4 benign tumors encountered in our study, 3 were Angiomyolipomas and one oncocytoma ,these findings were similar to the results of studies by Abdul gafoor et al 13 &Aiman et al. 3
9 cases showed associated findings in the adjacent renal parenchyma which included cortical cysts, chronic glomerulonephritis and chronic pyelonephritis predominantly indicating an ongoing chronic process.
Conclusion
Histological classification of renal neoplasia has undergone significant changes with various new entities with distinct clinical , pathological and molecular characteristics. Histologic subtype of RCC strongly correlates with prognosis, clinical implications and therapeutic strategies, underscoring the importance of accurate diagnosis. Attention to morphology and targeted immunohistochemical panel allow accurate classification of most RCCs. In others, cytogenetic and molecular findings, although expensive can establish the diagnosis. Our study provides an insight into various commonly encountered histomorphological patterns of kidney tumors and their associated features.
[D2] Acknowledgement : "Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed."
[D3] Conflict of Interest statement: The authors have no conflicts of interest to declare.
Source of Funding : NIL
[D4] Authors and their contribution: Nalini M, Jyothi A Raj, and Naveen S have contributed to the conception and design of the study and have authored the main manuscript. Shwetha B and Sakshi Barve have collected the clinical and pathological data. All authors have contributed to the literature review, editing, and approval of the final draft of the manuscript. All authors agree to be accountable for the content of this manuscript and its submission to the International Journal of Current Research and review.
Remove parenthesis of all the reference number in the main body of the manuscriptMention here acknowledgementMention here conflict of interest and source of funding. If there is no conflict of interest and source of funding then write NIL but mention itMention here individual author’s contribution.
TABLES
Table 1 : Distribution of neoplastic and non-neoplastic lesions.
References:
-
Alpers CE, Chang A. Robbins and Cotran Pathologic basis of disease. 9e South Asian edition. India: Reed Elsevier: 2014. Chapter 20, the Kidney: p 897-898.
-
Srigley JR, Zhou M, Allan R, Amin MB, Campbell SC, Chang A et al. Protocol for the Examination of Specimens from Patients with Invasive Carcinoma of Renal Tubular Origin. College of American Pathologists; June 2017. 15p. available from www.cap.org/cancerprotocols version : kidney 4.0.1.0
-
Aiman A, Singh K, Yasir M. Histopathological spectrum of lesions in nephrectomy specimens: A five-year experience in a tertiary care hospital. J Sci Soc 2013;40:148-54.
-
Sahni VA, Silverman SG. Biopsy of renal masses: when and why. Cancer Imaging. 2009;9(1):44.
-
Bray F, Ferlay J, Soerjomataram I, Siegal RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Cancer J Clin. 2018;68:394-424
-
Muglia VF, Prando A. Renal cell carcinoma: histological classification and correlation with imaging findings. Radiol Bras. 2015 Mai/Jun;48(3):166–174.
-
Hes O, Compérat EM, Rioux-Leclercq N, Kuroda N. The 2012 ISUP Vancouver and 2016 WHO classification of adult renal tumors: changes for common renal tumors. Diagnostic histopathology. 2016 Feb 1;22(2):41-6.
-
Udager AM, Mehra R. Morphologic, Molecular, and Taxonomic Evolution of Renal Cell Carcinoma - A Conceptual Perspective With Emphasis on Updates to the 2016 World Health Organization Classification. Arch Pathol Lab Med. 2016;140:1026–1037
-
Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright UM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs—Part A: Renal, Penile, and Testicular Tumours EUROPEAN UROLOGY70(2016)93–105
-
Vinay KS, Sujatha S. Histopathological Spectrum of Nephrectomy Specimens: Single Center Experience. Biomed J Sci&Tech Res 6(3)- 2018.
-
Reddy KD, Gollapalli SL, Chougani S, Sidagam S, Mohmmed AK, Bommanna A. (2016) A clinic-morphological spectrum of nephrectomy specimens-an experience from a tertiary care hospital. Int J Health Sci Res 6(11): 67-72
-
Bashir N, Bashir Y, Shah P, Bhat N, Salim O, Samoon N et al. Histopathological study of renal tumors in resected Nephrectomy specimens-An experience from teritary care centre. Nat J Med Res. 2015; 5(1):25-29.
-
Ghafoor AS. Kareem A, Bashar A. Hassawi, Ziyad Ahmed. Nephrectomy. A clinicopathological study. J Am Sci 2015;11(8):97-101
-
Narang VI, Garg BH, Walia AS, Sood NE, Malhotra VI. Histomorphological Spectrum of Nephrectomy Specimens-A Tertairy Care Centre Experience. National Journal of Laboratory Medicine. 2016 Apr;5(2):51-4.
-
Ibrahim SS, Dhanabalan RT, Sankari S, Shanmuganathan LK, Thandavarayan P, Ramalingam S et al. A histopathological review of nephrectomy specimens received in a referral center in South Tamil Nadu. Age. 2016;1(10).
-
Chaitra B, Prema LP, Tejeswini V, Haritha O, Anusha M. Histopathology of nephrectomy specimens: A ten year south Indian tertiary hospital-based study. J Diagn Pathol Oncol. 2018;4(3):232-236.
-
Ajmera S, Ajmera R (2017). Histopathological spectrum of lesions in nephrectomies- A five year study. Int J Sci Res 6(7): 44-46
-
Thaker BD, Singh K (2017). A histopathological review of Nephrectomy specimens Received in a Tertiary care hospital-A retrospective study. J Med Sci Cl Res 5(6): 23807-23810
-
Popat VC, Kumar MP, Udani D, Mundra MP, Vora DN, Porecha MM. A study on culprit factors ultimately demanding nephrectomy. Internet J Urol 2010;7(1):1-8.
-
Kuroda N, Ohe C, Kawakami F, Mikami S, Furuya M, Matsuura K et al. Clear cell papillary renal cell carcinoma: A review. Int J Clin Exp Pathol 2014;7(11):7312-7318
-
Srigley JR, Cheng L, Grignon DJ, Tickoo SK. Clear cell papillary renal cell carcinoma in chapter 1: Tumors of the kidney. Moch H, Humphrey PA, Ulbright TM, Reuter VE (Eds): WHO Classification of Tumours of the Urinary System and Male Genital Organs (4th edition) IARC: Lyon 2016. pp 40-41
-
Ross H, Martignoni G, Argani P. Renal Cell Carcinoma with Clear Cell and Papillary Features. Arch Pathol Lab Med. 2012;136:391–399
-
Shuch B, Bratslavsky G, Linehan WM, Srinivasan R. Sarcomatoid Renal Cell Carcinoma: A Comprehensive Review of the Biology and Current Treatment Strategies. The Oncologist 2012; 17: 46- 54
-
Liang X, Liu Y, Ran P, Tang M, Xu C, Zhu Y. Sarcomatoid renal cell carcinoma: a case report and literature review. BMC Nephrology (2018) 19: 84
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License