IJCRR - 13(7), April, 2021
Pages: 29-33
Date of Publication: 12-Apr-2021
Print Article
Download XML Download PDF
Antibiogram and Isolation of S. aureus from the Urinary Tract Infections: Comparison of Meca Gene Detection and Phenotypic Methods for Detection of Methicillin-Resistant S. aureus
Author: Basavaraj C. Metri, Jyothi P
Category: Healthcare
Abstract:Introduction: Urinary tract infections (UTIs) are one of the most common infectious diseases, and nearly 10% of people will experience a UTI during their lifetime. S. aureus is one of the most widely spread human pathogens. Objective: To detect Methicillin-resistant Staphylococcus aureus(MRSA) among S. aureus causing UTI. Objectives: To know the antibiotic sensitivity pattern of the isolates and comparison of mecA gene detection and phenotypic methods for the detec�tion of MRSA. Methods: This study conducted over 2 years from January 2017 to December 2018. The isolates were identified by standard protocol. All isolates were tested for antimicrobial susceptibility. MRSA was identified by Cefoxitin and Oxacillin disk diffusion method. mecA gene was detected by PCR. Results: UTI were reported more among females in the age group of 21 to 40 with a rate of 42%. Among the male patients, UTI was reported more in elderly patients with 50% of cases occurring between the age group of 40-60 years. Linezolid was found to be the most effective drug overall against S.aureus. The highest percentages of resistance were found for penicillin and pefloxa�cin. Cefoxitin and Oxacillin detected 19 and 15 isolates as MRSA respectively, the PCR detected mec A genes in 20 isolates. Conclusion: UTIs were more among young females patients and elderly male patients. PCR was the best method for the detec�tion of MRSA. Cefoxitin disc is the best alternatives for PCR for the identification of MRSA. Antibiotic sensitivity revealed MRSA were resistant to many antibiotics but were sensitive to tetracycline, gentamicin, vancomycin and linezolid.
Keywords: Urinary tract infections, S.aureus, MRSA, PCR, mecA gene
Full Text:
Introduction
Bacterial resistance to antibiotics increases mortality, the likelihood of hospitalization and the length of stay in hospital. Resistance is related to the increasing usage of antimicrobial agents; growing numbers of patients with impaired immunity; increasing instrumentation, and emphasis on cost control. Furthermore; it no longer remains the domain of Gram-negative bacteria. Antimicrobial usage may be controlled by antibiotic policies, but these can only be formulated if the antimicrobial susceptibility pattern of prevalent bacterial pathogens is known.1
Urinary tract infections (UTIs) are one of the most common infectious diseases, and nearly 10% of people will experience a UTI during their lifetime.2-4 The infections may be symptomatic or asymptomatic, and either type of infection can result in serious sequelae if left untreated. Klebsiella, Staphylococci, Enterobacter, Proteus, Pseudomonas, and Enterococci species are more often isolated from inpatients, whereas there is a greater preponderance of E. coli in an outpatient population. Corynebacterium urealyticum has been recognized as an important nosocomial pathogen. Anaerobic organisms are rarely pathogens in the urinary tract.5-8 Coagulase Negative Staphylococci are a common cause of urinary tract infection in some reports. Staphylococci saprophyticus tends to cause infection in young women of sexually active age.9,10
S. aureus is one of the most widely spread human pathogens. Considering the havoc it causes on life and subsequently on the economy, it became necessary to determine its incidence and antibiogram in our environment for adequate control and treatment.11 of infections caused by S. aureus can be one of the gratifying experiences in clinical practice. Survey of resistant patterns of microbes to drugs has shown a rise in the incidence of microbial resistance to most prescribed antibiotics. The study aimed to detect MRSA among S. aureus causing UTI and to know the antibiotic sensitivity pattern of the isolates in our hospital setting.
Materials and methods
Study design, setting
This study conducted in the Department of Microbiology, Shri B M Patil Medical College over 2 years from January 2017 to December 2018.
Sample collection
The samples included midstream urine specimen, catheterized urine samples, supra-pubic aspirates collected in sterile universal bottles. The urine specimens were transported to the bacteriology laboratory within 2 hours of collection.12
Statistical analysis
Values were expressed in terms of Mean ± SD. Analysis was done by using SPSS software version 16. P≤0.05 was considered statistically significant.
Microbiological analysis
All urine samples were examined by routine microscopic examination by the wet mount of urine sediment. All urine samples were cultured over routine culture media with a sterile standard loop. These plates were incubated at 37°C for 2 consecutive days. Culture results were interpreted according to the standard criteria.13 Cultures with more than three colonies were discarded, as contaminants 12 The isolates were identified by gram staining, colony morphology and standard biochemical tests catalase, slide and tube coagulase, mannitol salt agar test, phosphatase test.14
Antimicrobial susceptibility testing
All isolates were tested for antimicrobial susceptibility on Mueller Hinton agar by the standard disc diffusion method recommended by the Clinical and Laboratory Standards Institute (CLSI).15
Detection of MRSA
The Cefoxitin Disc Diffusion Test: the test was carried out on Mueller-Hinton agar by using a 30 μg cefoxitin disc. An interpretation was done using the Kirby-Bauer charts. An inhibition zone diameter of ≤ 21 mm was reported as methicillin resistant.16
The Oxacillin Disk Diffusion Method: The Oxacillin disk (1 μg) diffusion method was carried out on Mueller-Hinton agar which was supplemented with 4% NaCl to detect MRSA according to the CLSI guidelines. The isolates were considered as resistant when the diameter of inhibition was ≤10 mm.17
Genotypic detection of MRSA by PCR (mec A gene)
DNA Extraction Procedure was done by Modified Proteinase-K method.18,19 MRSA strains were amplified by conventional PCR. Following a set of PCR primers were used which were specific to Methicillin-resistant S.aureus.20
Forward Primer: 5'- TGC TAT CCA CCC TCA AAC AGG -3' Reverse Primer: 3'-AAC GTT GTA ACC ACC CCA AGA -5'
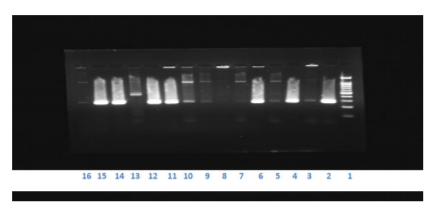
Figure 1: Results of mecA gene (left to right), Lane 1: Molecular weight marker, Lane 2: MRSA ATCC 43300, Lane 3: MSSA ATCC 25923, Lane 4, 6, 11,12,14 and 15: MRSA isolates from clinical samples(280 BP), Lane 5,7-10,13,16: MSSA isolates from clinical sample.
We had chosen a primer set that gives an amplified product of size 280 base pair. So the well which gives a DNA band of 280 base pair is considered positive, whereas the well which does not have any DNA band is indicated as negative ( Figure 1).
Results
Discussion
UTI(UTIs) are one of the most prevalent extra-intestinal bacterial infections. Nowadays, it represents one of the most common diseases encountered in medical practice affecting people of all ages from the neonate to the geriatric age group.21,22
In our study, the UTI was reported more among the age group of 21 to 40 with a rate of 42%. the findings are in agreement with the study conducted by Inaba et al,.23 and El-Sweih et al,.24 this can be explained by the fact the structure of the female's urethra and vagina makes it susceptible to trauma during sexual intercourse and pregnancy and or childbirth.24,25
S. aureus UTI more often occurs in urinary-catheterized and pregnant individuals. The majority of S. aureus UTI isolates are methicillin-resistant and S. aureus bacteriuria is associated with subsequent development of invasive infection.26-28 Like S. saprophyticus, S. aureus also encodes an active urease enzyme. Two nickel ABC-transporters (Opp2 and Opp5a) have been identified as necessary for urease activity in vitro. These, along with a third ABC-transporter that imports nickel and cobalt when zinc is depleted, are both involved in UTI colonization and virulence in a mouse model.29-32
Urinary tract infection is one of the most important causes of morbidity in the general population and is the second most common cause of hospital visits.32 Among the male patients, UTI was reported more in elderly patients with 50% of cases occurring between the age group of 40-60 years. Our finding is in agreement with a study conducted by Das et al.33
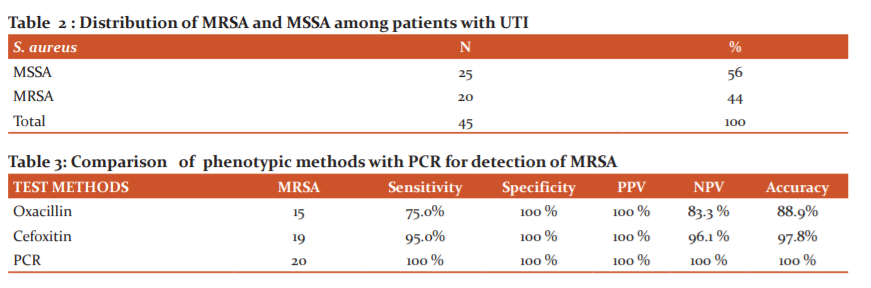
With advancing age, the incidence of UTI increases in men due to prostate enlargement and neurogenic bladder.34 Recurrent infections are common and can lead to irreversible damage of the kidneys, resulting in renal hypertension and renal failure in severe cases.33 UTIs have been reported to be the majority caused by Gram-negative bacteria with E. coli being the most prevalent. However, there is an increasing prevalence of S. aureus as a UTIs etiological agent with an alarming rate of developing antimicrobial resistance (Table 1 and 2).35
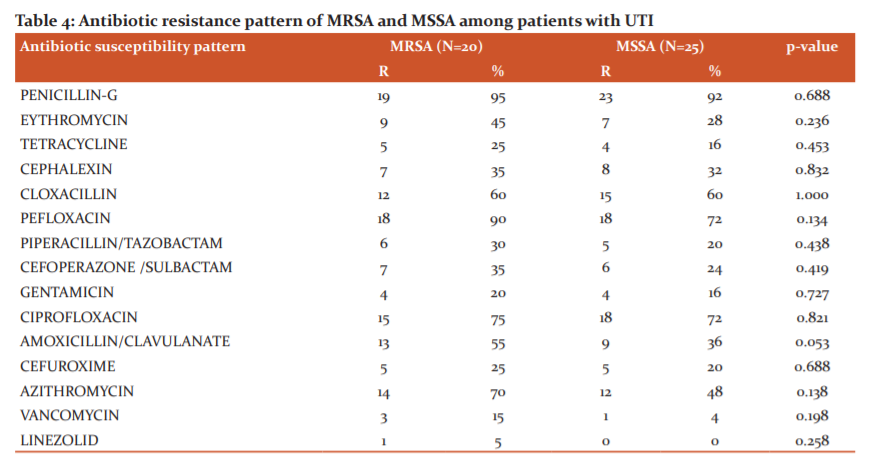
Linezolid was found to be the most effective drug overall against S. aureus followed by vancomycin, tetracycline, gentamycin and cefuroxime. The highest percentages of resistance were found for penicillin, pefloxacin. these results are basically in agreement with other studies carried out around the world. Our findings illustrate that antimicrobial therapy needs to be selected based on actual culture findings and antimicrobial sensitivity patterns of isolates (Tables 3 and 4).
Antibiotic susceptibility pattern revealed a high resistance to routinely used antibiotics. Resistance to quinolones I,e. ciprofloxacin and pefloxacin were high in this study. This is comparable to the study done by Sanjana et al,36 in Nepal. Majumder et al,.37 also revealed that resistance to various antibiotics with methicillin-resistant strains was s higher in comparison to methicillin-sensitive isolates. Factors responsible for drug resistance in MRSA are as follows. Antibiotics are available without a prescription at drug stores or even at general stores and injudiciously used in communities, animal husbandries, and fisheries and use of allopathic drugs by traditional practitioners.38
In our study, the mecA gene PCR detected 20 isolates as MRSA and the 25 isolates as MSSA. Detection of mecA gene is considered the best method for MRSA confirmation.The accurate and early determination of methicillin resistance is of key importance in the prognosis of infections caused by S. aureus.39 This higher sensitivity to cefoxitin can be explained by the increased expression of the mecA-encoded protein PBP2a, cefoxitin being an inducer of the mecA gene.39 Our study reveals that cefoxitin disc is better than oxacillin disc for the detection of methicillin resistance.
Conclusion
To conclude, we found that UTIs were more among young females patients and elderly male patients. PCR was the best method for the detection of MRSA but in peripheries where it is not available Cefoxitin disc diffusion test is the best alternatives for PCR for the identification of MRSA. Antibiotic sensitivity revealed MRSA were resistant to many antibiotics but were sensitive to tetracycline, gentamicin, vancomycin and linezolid.
Acknowledgements:
The authors acknowledge the immense help received from the scholars whose articles are cited and included in references to this manuscript. The authors are also grateful to authors/editors/publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
Conflict of interest: Nil
Source of Funding: Nil
References:
-
Winstanleya TG, Limba DI, Eggingtona R, Hancock F. A 10 year survey of the antimicrobial susceptibility of urinary tract isolates in the UK: the Microbe Base project. J Antimicrob Chemother 1997;40:591–594.
-
Hoberman A, Wald ER. UTIin young febrile children. Pediatr Infect Dis J 1997;16:11-17.
-
Delanghe J, Kouri TT, Huber AR, Hannemann-Pohl K, Guder WG, Lun A, et al,. The role of automated urine particle flow cytometry in clinical practice. Clin Chim Acta 2000;301:1-18.
-
Hryniewicz K, Szczypa K, Sulikowska A, Jankowski K, Betlejewska K, Hryniewicz W. Antibiotic susceptibility of bacterial strains isolated from UTIin Poland. J Antimicrob Chemother 2001;47:773-780.
-
Farajnia S, Alikhani M Y, Ghotaslou R, Naghili B, Nakhlband A. Causative agents and antimicrobial susceptibilities of UTI in the northwest of Iran. J Infect Dis 2009;13:140-144.
-
Bronsema DA, Adams JR, Pallares R. Secular trends in rates and aetiology of nosocomial UTI at a university hospital. J Urol 1993;150:414-416.
-
Soriano F, Aguado JM, Ponte C. Urinary tract infection caused by Corynebacterium group D2: Report of 82 cases and review. Rev Infect Dis 1990;12:1019-1034.
-
Jacobs LG. Fungal UTI in the elderly: Treatment guidelines. Drugs Aging 1996;8:89-96.
-
Schneider PF, Riley TV. Staphylococcus saprophyticus urinary tract infections: Epidemiological data from Western Australia. Eur J Epidemiol 1996;12:51-54.
-
Amin M, Mehdinejad M, Pourdangchi Z. Study of bacteria isolated from UTI and determination of their susceptibility to antibiotics. Jundishapur J Microbiol 2009;2(3):118-123.
-
Akortha EE, Ebadin OK. Incidence and antibiotic susceptibility pattern of Staphylococcus aureus amongst patients with urinary tract infection (UTI) in UBTH Benin City, Nigeria. Afr J Biotech 2008;7(11):1637-1640.
-
Collee JG, Duguid JP, Fraser AG, Marmion BP, Simmons A. Laboratory strategy in diagnosis of infective syndromes. In: Collee JG, Duguid JP, Fraser AG, Marmion BP, Simmons A (editors). Mackie and McCartney Practical Medical Microbiology, 14th ed. London: Churchill Livingstone ; 1996:53-94.
-
Cruickshank R, Duguid JP, Marmion BP. Tests for identification of bacteria. In: Medical Microbiology. 12th ed. London: Churchill Livingstone; 1975:170-89.
-
Forbes BA. Sahm DF, Weissfeld AS. Bailey and Scott's Diagnostic microbiology, 12th edition, Mosby Elsevier; 2007:842-55.
-
The Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility testing, twenty-first informational supplement, M100-S21, Clinical and Laboratory Standards Institute, 2011.
-
Isenberg HD, editor. Clinical microbiology procedures handbook. 2nd ed. Washington DC: ASM Press; 2004:192-98.
-
Brown DF, Edwards DI, Hawkey PM, Morrison D, Ridgway GL, Towner KJ, et al.Guidelines for the laboratory diagnosis and susceptibility testing of methicillin-resistant Staphyloccocus aureus. J Antimicrob Chemother 2005;56:1000-1018.
-
Rao Venkatakrishna I, Bhat Kishore G, Kugaji Manohar S, Manjula PV. Detection of methicillin resistance in Staphylococcus aureus: Comparison of Disc diffusion and MIC with mecA gene detection by PCR. Int J Pharm Bio Sci 2011;1:518-21.
-
Vanpelt E, Belkum VAV, Hays JP. Principles and Technical Aspects of PCR amplification. Springer 2008;34.
-
Boucher HW, Corey GR. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin Infect Dis 2008;46(Suppl 5):S344–S9.
-
Kunin CM. UTI in females. Clin Infect Dis 1994;18:1-10.
-
Gupta K, Scholes D, Stamm WE. Increasing prevalence of antimicrobial resistance among uropathogens causes acute uncomplicated cystitis in women. J Am Med Assoc 1999;281:736-738.
-
Inabo HI, Obanibi HBT. Antimicrobial susceptibility of some urinary tract clinical isolates to commonly used antibiotics. Afr J Biotech 2006;5:487-489.
-
Al-Sweih N, Jamal W, Rotimi VO. Spectrum and antibiotic resistance of uropathogens isolated from hospital and community patients with UTI in two large Hospitals in Kuwait. Med Princ Pract 2005;14:401-407.
-
Manikandan S, Ganesapandian S, Singh M, Kumaraguru AK. Emerging of Multidrug Resistance Human Pathogens from Urinary Tract Infections. Curr Res Bacteriol 2011;4: 9-15.
-
Muder RR, Brennen C, Rihs JD, Wagener MM, Obman A, Stout JE, et al. Isolation of Staphylococcus aureus from the urinary tract: association of isolation with symptomatic urinary tract infection and subsequent staphylococcal bacteremia. Clin Infect Dis 2006;42:46–50.
-
Baraboutis IG, Tsagalou EP, Lepinski JL, Papakonstantinou I, Papastamopoulos V, Skoutelis AT, et al. Primary Staphylococcus aureus urinary tract infection: the role of undetected hematogenous seeding of the urinary tract. Eur J Clin Microbiol Infect Dis 2010;29:1095-1101.
-
Gilbert NM, O’Brien VP, Hultgren S, Macones G, Lewis WG, Lewis AL. Urinary tract infection as a preventable cause of pregnancy complications: opportunities, challenges, and a global call to action. Glob Adv Health Med 2013;2:59–69.
-
Hiron A, Posteraro B, Carrière M, Remy L, Delporte C, La Sorda M, et al. A nickel ABC-transporter of Staphylococcus aureus is involved in urinary tract infection. Mol Microbiol 2010;77:1246–1260.
-
Remy L, Carrière M, Derré-Bobillot A, Martini C, Sanguinetti M, Borezée-Durant E. The Staphylococcus aureus Opp1 ABC transporter imports nickel and cobalt in zinc-depleted conditions and contributes to virulence. Mol Microbiol 2013;87:730–743.
-
Kline KA, Lewis AL. Gram-Positive Uropathogens, Polymicrobial Urinary Tract Infection, and the Emerging Microbiota of the Urinary Tract. Microbiol Spectr 2016; 4(2).
-
Ronald AR, Pattulo MS. The natural history of urinary infection in adults. Med Clin North Am 1991;75:299-312.
-
Das RN, Chandrashekhar TS, Joshi HS, Gurung M, Shrestha N, Shivananda PG. Frequency and susceptibility profile of pathogens causing UTI at a tertiary care hospital in western Nepal. Singapore Med J 2006;47(4):281.
-
Liperky BA. Urinary tract infection in men: epidemiology, pathophysiology, diagnosis and treatment. Ann Intern Med 1989;111:138-150.
-
Onanuga A, Awhowho GO. Antimicrobial resistance of Staphylococcus aureus strains from patients with UTI in Yenagoa, Nigeria J Pharm Bio Sci 2012;4:226-230.
-
Sanjana RK, Shah R, Chaudhary N, Singh YI. Prevalence and antimicrobial susceptibility pattern of methicillin-resistant Staphylococcus aureus (MRSA) in CMS-teaching hospital: a preliminary report. J College Med Sci Nepal 2010;6:1-6.
-
Majumder D, Bordoloi JS, Phukan AC, Mahanta J. Antimicrobial susceptibility pattern among methicillin-resistant staphylococcus isolates in Assam. Indian J Med Microbiol 2001;19:138-140.
-
Metri BC, Peerapur BV, P Jyothi. Comparison of antimicrobial resistance pattern of hospital-and community-acquired Methicillin-resistant Staphylococcus aureus. J Chem Pharm Res 2014;6:201-205.
-
Anand KB, Agrawal P, Kumar S, K Kapila K. Comparison of cefoxitin disc diffusion test, oxacillin screen agar, and PCR for mecA gene for detection of MRSA. Indian J Med Microbiol 2003;27:27-29.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License