IJCRR - 8(1), January, 2016
Pages: 16-19
Print Article
Download XML Download PDF
A RARE CASE OF PERSISTENT MULLERIAN DUCT SYNDROME PRESENTING AS HERNIA UTERO INGUINALIS
Author: N. Murugesan, M. S. Viswanathan, Lakshmanan S., Pankaj Surana, R. Anbhazakan
Category: Healthcare
Abstract:The aim of presentation of this rare case of persistent mullerian duct syndrome is to outline management of such case when encountered unexpectedly while operating on obstructed inguinal hernia. More so this possibility should be kept in mind while operating on a case of cryptorchidism. PMDS is a rare condition often missed in a casual USG done in emergency this leads to confusion to surgeon while operating. Left behind gonads pose a risk of malignant degeneration if ignored and hence will need further management. Presence of risk of infertility after definitive treatment especially in young patients precludes immediate surgical removal of gonads in such patients. Proper informed consent prior to such procedure is important as is advise on future fertility.
Keywords: Persistent mullerian duct syndrome (PMDS), Pseudohermaphroditism, Transverse testicular ectopia (TTE), Cryptorchidism, Hernia utero inguinalis
Full Text:
INTRODUCTION Persistent Mullerian duct syndrome (PMDS) is a rare autosomal recessive disorder which could present as male pseudohermaphroditism in which Mullerian duct derivatives are seen in a male patient. This syndrome is characterized by the persistence of Mullerian duct derivatives (i.e. uterus, cervix, fallopian tubes and upper two thirds of vagina) in a phenotypically and karyotypically male patient.The syndrome is caused either by an insufficient amount of Mullerian inhibiting factor (MIF) or due to insensitivity of the target organ to MIF.
CASE REPORT A 30-year-old male presented to out patient department for a right inguinoscrotal swelling, which was present since 1 year and associated with pain and irreducibility for the past 1 week. Detailed history revealed an associated infertility and he has been married since 4 years. He reported normal sexual activity and had well developed secondary sexual characters. No significant family or personal history was noted. Abdominal examination revealed tense, tender, irreducible right inguinoscortal swelling with absent cough impulse. There were with no signs of intestinal obstruction or strangulation. On genital examination, he had normal male genitalia with normal appearing fully developed penis along with left sided non palpable testis and undeveloped scrotum. The testis on right side could not be appreciated due to tenseness of the hernia. Urgent ultrasound to evaluate revealed absent left testis in left inguinal region and right testis in right inguinal hernia with some ill defined mass in the hernial sac not unlike intestine or bowel. Rest of his lab investigations, ECG and CXR were within normal limits. With a clinical diagnosis of right sided irreducible inguinal hernia with absent testis on left side.
He was taken up for emergency surgery under spinal anesthesia for primarily relieving obstructed inguinal hernia. The exploration of opposite absent left testis was deferred to later date after proper imaging studies. Intraoperatively it was noted that the right hernial sac contained an underdeveloped uterus and bilateral fallopian tube with fimbria like structures. There were two gondal structures on either sides of fallopian tubes which appeared ovoid with smooth surface like testis. There was no evidence of strangulation and the tight neck of hernial sac was released and contents reduced. In order to evaluate, explain and to obtain consent from the patient, these abnormal mullerian structures were not removed in that first setting of open surgery. Mesh repair was done in standard way. His post operative recovery was uneventful. Further evaluations were done to confirm the type of abnormality.
a. Karyotyping was done that revealed 46XY genotype male
b. Tumor markers beta HCG and alpha fetoprotein were within normal limits.
c. CECT revealed uterus and fallopian tubes of almost normal size. Lower end of uterus ended in a cord like tissue that could be traced up to prostate. Bilateral hypoplastic testis were noted intraabdominally. No ovarian tissue was found. There were no abnormalities were noted in kidneys, ureters and bladder.
d. Semen analysis was azoospermic
e. Hormonal analysis revealed normal level of testosterone, oestrogen and progesterone for male
Patient and his wife were appraised and counselled about his condition. The reason for their infertility was explained. The need for removal of the abnormal uterus fallopian tubes and intra abdominal hypoplastic testes was explained. Appropriate consents were obtained for laparoscopic approach after explaining the risks and complications. Three months later under general anaesthesia the persistent mullerian structures uterus, fallopian tubes, vaginal tube up to its entry into prostate and both testis were removed laparoscopically by standard technique as used for laparoscopic hysterectomy with bilateral salpingooophorectomy. Intraoperatively the cervix portion continued as a thick cord of like structure going deep in rectovescical plane. The dissection was taken deep and it was divided close to level of prostate. Post operative course was uneventful. Histopathology of the specimen revealed normal endometrial, myometrial and cervix and fallopian tube histology with normal seminiferous tubules lined by sertoli cells and foci of leydig cells. No epididymal structures found. And there was no evidence of spermatogenesis. Patient was regularly followed and is presently on testosterone replacement therapy. He was advised ART with donor sperm or adoption for infertility.
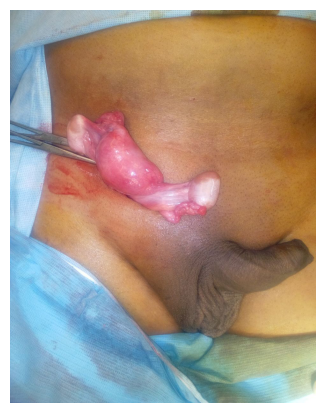


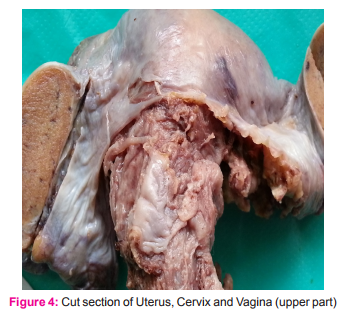
DISCUSSION Müllerian (paramesonephric) ducts and wolffian (mesonephric) ducts are the anlagen of the female and male reproductive tracts, respectively. In the XY fetus, the testis differentiates by the end of the seventh gestational week. Sertoli cells begin to secrete AMH, which is responsible for the regression of the Müllerian ducts. The AMH binds to a specific Type II serine-threonine kinase transmembrane receptor (AMHR-II). Human AMH gene localized near the tip of Chromosome 19, AMHR2 gene is located on 12q13. The type of persistent Mullerian duct syndrome caused by mutation in the AMH gene will be referred to as Type I, that which forms due to mutation in the AMH receptor (AMHR) will be designated as Type II.[1] In 45%, a mutation of the anti-mullerian hormone (AMH) gene was detected; in 39% mutation of the Type II receptor of AMH was detected; in 16% the cause is unknown.
Transverse testicular ectopia (TTE) or crossed testicular ectopia is a rare form of testicular ectopia. It was first reported by Von Lenhossek in 1886 [2]. More than 100 cases have been reported in the literature [3]. Several theories have been reported to explain the genesis of TTE. Berg [4] proposed the possibility of the development of both testes from the same genital ridge. Kimura [5] concluded that if both vasa deferentia arose from one side, there had been unilateral origin but if there was bilateral origin, one testis had crossed over. Gupta and Das [6] postulated that adherence and fusion of the developing Wolffian ducts took place early, and that descent of one testis caused the second one to follow. An inguinal hernia is invariably present on the side to which the ectopic testis has migrated.
On the basis of the presence of various associated anomalies, TTE has been classified into 3 types: Type 1, accompanied only by hernia (40% to 50%); type 2, accompanied by persistent or rudimentary Mullerian duct structures (30%); and type 3, associated with disorders other than persistent Mullerian remnants (inguinal hernia, hypospadias, pseudohermaphroditism, and scrotal abnormalities) (20%). According to that classification, our case was type 1/2 TTE. TTE associated with fused vas deferens is extremely rare. This condition may hinder the testis from being placed into the scrotum during orchidopexy [7].
The clinical presentation generally includes an inguinal hernia on one side and a contralateral or sometimes a bilateral cryptorchidism [8], [9]. Usually, the correct diagnosis is not made before surgical exploration, like our case, and it is revealed during herniotomy [9]. The diagnosis of TTE can be made preoperatively by using ultrasonography [10] by an experienced sonologist. Patients with TTE are at increased risk of malignant transformation. In fact, the overall incidence of malignant transformation of gonads is 18% [11]. There have been reports of embryonal carcinoma [12], seminoma, yolk sac tumor [13], and tera- toma [11]. Walsh et al. [14] in their study concluded that testicular cancer was nearly 6 times more likely to develop in cryptorchid cases whose operations were delayed until after age 10 to 11 years. Wood et al. [15] in their study showed that risk of malignancy in undescended testicles decreased if their orchidopexy performed before ages 10 to 12 years.
Orchidectomy of ectopic testis should be done, because orchidopexy offers only limited protection against future malignancy if performed after two years of age.[16] Manassero et al reported development of mixed germ cell tumor 18 years after bilateral orchidopexy.[17] Most are known to be infertile but it is preferable to remove ectopic testis, as it is prone for malignancy. If this is necessary on both sides, there is the additional problem of lifelong testosterone substitution which requires efficient patient monitoring and good patient compliance. In cases where this cannot be achieved, compromises, such as temporarily delayed orchidectomy, may be considered.[18] Testis, vas and epididymis are closely adherent running along the uterus and fallopian tubes. This gives rise to difficulty in separating the gonads and the vas without damage. Different surgical methods have been described for safe surgery.
There have been at least three documented reports of adenocarcinoma in the mullerian duct remnants. So, contrary to previous suggestions, now it is recommended to remove the persistent mullerian derivatives. The patient or his family should be completely informed of the diagnosis, the surgical options and the need for long-term follow-up. Finally, genetic counseling must be offered to the patient or his parents because of the possible chromosomal origin of the syndrome.
CONCLUSION This is a rare presentation of persistent mullarian duct syndrome reported few and far in literature. The surgeon operating on inguinal hernia in a cryptorchid patient, need to be aware of management of this condition, when encountered in an emergency situation. The future fertility of the patient need to be kept in mind and counselling before performing the definitive surgery is essential to prevent future litigation. Specimen harvest of sperms and storage for future use could be planned inappropriate setup if the development of testis seems adequate. Laparoscopic approach is ideal and minimally invasive way for definitive removal of remnant mullerian structures.
References:
1. Imbeaud S, Faure E, Lamarre I, Mattei MG, Clemente N, Tizard R, et al. Insensitivity to anti-mullerian hormone due to a mutation in the human anti-Mullerian hormone receptor. Nat Genet. 1995;11:382–8. [PubMed: 7493017]
2. Von Lenhossek MN. Ectopia testis transversa. Anat Anz.1886;1:376-81.
3. Fourcroy JL, Belman AB. Transverse testicular ectopia with persistent Müllerian duct. Urology. 1982; 19(5):536-8. DOI: 10.1016/0090-4295(82)90614-8
4. Berg AA. Transverse ectopy of the testis. Ann Surg. 1904;40:223
5. Kimura T. Transverse ectopy of the testis with masculine uterus. Ann Surg. 1918;68(4):420-5. DOI: 10.1097/00000658- 191810000-00009
6. Gupta RL, Das P. Ectopia testis transversa. J Indian Med Assoc. 1960;16:35:547-9.
7. Chacko JK, Furness PD 3rd, Mingin GC. Presentation of fused vas deferens. Urology. 2006; 67(5):1085.e17-8. DOI:10.1016/j. urology.2005.11.056
8. Feizzadeh Kerigh B, Mohamadzadeh Rezaei M. Crossed testicular ectopia: a case report. Urol J. 2005;2(4):222-3. Available from: http://www.urologyjournal.org/index.php/uj/article/ view/229/226
9. Acikalin MF, Pasaoglu O, Tokar B, Ilgici D, Ilhan H. Persistent Mullerian duct syndrome with transverse testicular ectopia: A case report with literature review. Turk J Med Sci. 2004;34:333- 6.
10. Nam YS, Baik HK, Kim SJ, Lee HK, Park HK. Transverse testicular ectopia found by preoperative ultrasonography. J Korean Med Sci. 1998; 13(3):328-30.
11. Berkmen F. Persistent müllerian duct syndrome with or without transverse testicular ectopia and testis tumours. Br J Urol. 1997; 79(1):122-6.
12. Melman A, Leiter E, Perez JM, Driscoll D, Palmer C. The influence of neonatal orchiopexy upon the testis in persistent Müllerian duct syndrome. J Urol. 1981;125(6):856-8.
13. Eastham JA, McEvoy K, Sullivan R, Chandrasoma P. A case of simultaneous bilateral nonseminomatous testicular tumors in persistent müllerian duct syndrome. J Urol. 1992;148(2 Pt1):407-8.
14. Walsh TJ, Dall’Era MA, Croughan MS, Carroll PR, Turek PJ. Prepubertal orchiopexy for cryptorchidism may be associated with lower risk of testicular cancer. J Urol. 2007; 178(4 Pt1):1440-6. DOI: 10.1016/j.juro.2007.05.166
15. Wood HM, Elder JS. Cryptorchidism and testicular cancer: separating fact from fiction. J Urol. 2009; 181(2):452-61. DOI:10.1016/j.juro.2008.10.074
16. Berkmen F. Persistent mullerian duct syndrome with or without transverse testicular ectopia and testis tumors. Br J Urol. 1997;79:122–6. [PubMed: 9043511]
17. Manassero F, Cuttano MG, Morelli G, Salinitri G, Spurio M, Selli C. Mixed germ cell tumor after bilateral orchidopexy in persistent mullerian duct syndrome with transverse testicular ectopia. Urol Int. 2004;73:81–3. [PubMed: 15263798]
18. Buchholz NP, Biyabani R, Herzig MJ, Ali A, Nazir Z, Sulaiman MN, et al. Persistent mullerian duct syndrome. Eur Urol. 1998;34:230–2. [PubMed: 9732199]
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License