IJCRR - 13(5), March, 2021
Pages: 58-63
Date of Publication: 03-Mar-2021
Print Article
Download XML Download PDF
Qualitative and Quantitative Evaluation of Trace Elements in Amaranthaceae Family Medicinal Plant Using ICP-MS
Author: Malla Balakrishna, Pondala Seetharm
Category: Healthcare
Abstract:Introduction: Traditional medicine is an inseparable part of Indian culture. India has had a rich ethnobotanical heritage. Though much work has been performed to isolate organic compound, a little attention has been given to its inorganic elements. Some inorganic elements play an important role in various physiological processes involved in human health. Elements in excess or deficiency cause several diseases. Objective: To investigate the profile of certain trace elements in C. prostrate. The qualitative and quantitative analysis of trace elements in this plant reveals its herbal properties. Methods: Plant samples were roots, leaves and seeds collected from Srikakulam, Andhra Pradesh and taken for the analysis with the ratio of 1:1:1. IPC-MS has been used to evaluate the composition of liquid and allow high-sensitivity investigation of several metal elements which can be found as the trace. Results: This investigation of plant extracts has been done by using ICP-MS as it is powerful, accurate, fast and sensitive analytical technique. The study gives the presence of various concentrations of important trace elements which are having an essential role in the metabolism of the human body. The analysis results twenty trace elements including Li, Be, Al, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, As, Se, Rb, Sr, Ag, Cs, Ba and Tl. Conclusion: The present investigation deals with trace elements concerning C. prostrate medicinal plant reveals that the elements have significant roles in fighting human ailments and diseases.
Keywords: Inductively coupled plasma mass spectrometry (ICP-MS), Trace elements, Medicinal plants, C. prostrate, Essential metal ions
Full Text:
Introduction
Nature has provided us with herbals and medicines under different climatic conditions; Medicinal plants and herbals commonly used for human therapy Medicinal plants play a crucial role in traditional medicine and are widely consumed as home remedies. As per the WHO study, most of the world population relies on traditional medicine for their primary home treatment. Pain and inflammation are the two important factors on which present researchers concentrating more. The new drugs to be prepared with fewer effects to avoid side effects caused by non-steroidal anti-inflammatory drugs (NSAIDs) and opioids.1 The main focus of research on medicinal plants is due to their abundance and ease of access.
C. prostrata is straggling to the more or less erect annual herb of up to 1 m long and is widely distributed in tropical Africa, Asia, Australia and tropical America. The young foliage is often coloured red with burred and adhesive fruits. The plant is a weed of cultivated land, waste places as well as forest margins. In Ivory Coast, the sap is applied to sores and chancres and used as ear drops for otitis and headache while leafy twigs, inflorescences and seeds pulped into a paste with or without clay are used on sores, burns and fractures. In Nigeria and Cameroon, it is used in the treatment of articular rheumatism and dysentery while in Gabon, it is used in treating eye troubles, wounds and urethral discharges.2 In Traditional therapy, plant extracts are commonly used to control disorders. Due to its non-toxic nature and fewer side effects and high availability, people are using herbal medicine. Medicinal plants have a high potential of the healing capacity of diseases due to the presence of trace elements which are having a significant role in curing diseases.3 However, it is also known that higher concentration of trace elements in medicinal plants are responsible for their toxicity.
The essential and trace elements were available in medicinal plants absorbed into the human body by the consumption of herbal medicine. Due to the slight range between deficiency and toxicity of different elements for the human body, it is difficulty to the adequate dosage and health guidelines for usage of herbal medicine. Geochemical features of the soil govern the accumulation of macro and trace elements in the medicinal plants. Furthermore, Elements accumulated into plants from their aquatic and aerial environment and allow some plants used as bio monitors.4-8 In the present study, elemental analysis of, C. prostrate medicinal plants was carried out using inductively coupled plasma mass spectrometry (ICP-MS) technique.
MATERIALS AND METHODS
Experimental Details
Sampling
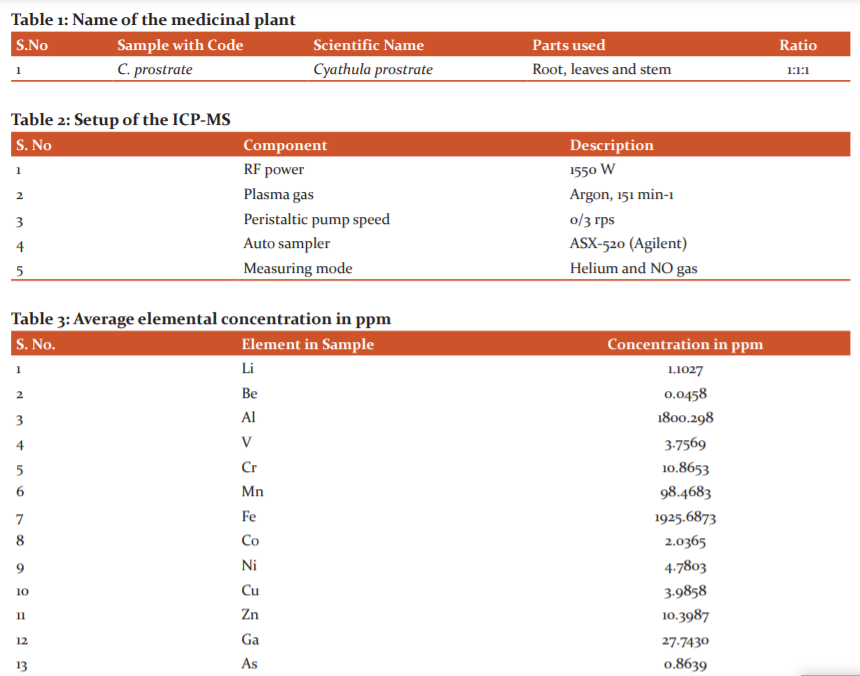
C. Prostrate was collected from premises of Srikakulam, Andhra Pradesh. The roots, leaves and stem parts collected from the plant, rinsed well with water to remove foreign materials deposited on them, then they are treated with ethanol for removal of surface contamination and finally washed with deionized water several times. All the parts allowed to dry under shade in the lab for one day and dried using the oven at 40 oC to make them crispy and finally pulverized separately using a blender. The powdered parts of plant materials allowed to room temperature for drying purpose and kept away from direct sunlight in closed plastic containers for further analysis. All the chemicals and solvents used in the present investigation were analytical grade. The sample containing roots, leaves and seeds were taken for the analysis with the ratio of 1:1:1. The details of the plant parts are shown in Table 1.
ICP-MS
ICP-MS is a rapid, powerful, accurate and sensitive technique used for trace elemental analysis of liquid samples and it can analyse more than 100 samples daily. It has been used to evaluate the composition of liquid and allow high-sensitivity investigation of several metal elements which can be found as the trace. The number of various elements present in the samples determined using a 7700 series ICP-MS (Agilent Technologies, USA). The ICP-MS was calibrated using MERCK XVII multi-element ICP-MS calibration standards (Merck KGaA, Germany), which was diluted with 3% nitric acid (HNO3). The setup of the ICP-MS is summarized in Table 2.
Digestion Procedure of Samples
1 gm of each sample (1:1:1 weight ratio of various parts of the plant) was digested in a nitric acid/Perchloric acid (6:1) using wet digestion method by heating slowly on a hot plate until white residue was obtained. The residue dissolved in 0.1 N Nitric acid and volume was made upto10 ml. The digested sample was analyzed ICP-MS Instrument.
RESULTS AND DISCUSSION
Twenty-elements (Li, Be, Al, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, As, Se, Rb, Sr, Ag, Cs, Ba and Tl) were identified in these medicinal plants. The concentration of each element in ppm was presented in Table 3. The concentration of Iron (Fe) reported higher followed by Aluminium (Al). The anti-inflammatory characteristic of this plant may be due to the presence of Zn with noticeable concentration. The list of macro and microelements determined by using ICP-MS technique and their concentration were given in Table 3.
The metal concentrations (ppm) in the C. prostrate are given in Table 3. The concentration of trace elements ranging from 0.0458 ppm of Beryllium (Be) to 1925.6873 ppm of iron (Fe). In the present study Iron (Fe) has reported the highest concentration in the medicinal plant followed by Aluminium (Al), Manganese (Al), Strontium (Sr), and Barium (Ba). The trace elements with less concentration in the medicinal plant are Beryllium (Be), Caesium (Cs), Thallium (Tl), Arsenic (As). Many therapeutic activities of this plant may be attributed and correlated to the presence of these potential trace elements. Deficiencies of these elements may cause different diseases. Zn has been reported to have beneficial effects on atherosclerotic patients as compared to normal (controls). The presence of Zinc (Zn) in the plant shows anti-inflammatory characteristic and may be correlated with its anticancer property. Copper (Cu) and Zinc (Zn) both elements are required in the growth and proliferation of normal cells. Zn concentration decreases in cancer patients whereas Cu concentration increases.9
Some are the heavy metals which cause serious health disorders when consumed by animals. According to the World Health Organization, skin cancer is induced because of long-term exposure to Arsenic. Beryllium causes pneumonia, lung disorders, cardiovascular damage and allergy. Lithium is a trace mineral most effective in mental health due to its neuroprotective potential, its deficiency influences common mental illness and social ills.
Cobalt (Co)
Cobalt is the main part of vitamin B-12 and helps to make DNA and blood cells. This is an essential element and occurs in inorganic and organic forms. The inorganic form of Cobalt is highly essential for the human body. Its excess or deficiency causes unfavourable conditions. The organic form of cobalt is present in green parts of plants, fish, cereals, and water.10, 11 In the present study, the sample contains 2.0365 ppm of Cobalt (Table 3 and Figure 1).
Arsenic (As)
Arsenic is highly toxic in its inorganic form, long term exposure to inorganic Arsenic through food can lead to poisoning. In the present study, the sample contains 0.8639 ppm of Arsenic (Table 3 and Figure 1).
Selenium (Se)
Selenium is an essential micronutrient for humans and animals when it takes in excessive leads to toxicity. Se is mainly available in plants as a source of dietary Se, but essentiality of Se for plants is still controversial. However, see at low doses protect the plants from a variety of abiotic stresses such as cold, drought, desiccation, and mental stress. In the present study, the sample contains 0.1854 ppm of Selenium (Table 3 and Figure 1).
Rubidium (Rb)
The typical daily dietary intake of Rb is expected to be 1–5 mg. Rb is highly present in fruits and vegetables. Rb is a relatively nontoxic element and has not shown toxicological concern from the nutritional point of view.12 In the present study, the sample contains 14.7367 ppm of Rubidium (Table 3 and Figure 1).
Strontium (Sr)
Since Sr is chemically similar to calcium it is taken up from the soil by fruits and vegetables. Assuming a reference body weight of 70 kg, the typical daily Sr exposure is 0.046 mg/kg of body weight. Extremely high Sr uptake can disrupt bone development and cause lung cancer. But this effect can only occur when Sr uptake is in the thousands of mg/kg range.13 Sr levels in our samples were not high enough to be able to cause these effects. In the present study, the sample contains 79.3409 ppm of Strontium (Table 3 and Figure 1).
Aluminium (Al)
The intake of Aluminium into the human body through various systems like lungs and skin, the accumulation of aluminium in the body causes situations such as neurological disorders, hyper alumina, dialysis encephalopathy and anaemia.14 It is suggested that the intake of Al is 140 mg/day, which has less threat.15 As per EFSA 10 mg of Al per day is possible accumulation.16 In the present study, the sample contains 1800.298 ppm of Aluminium (Table 3 and Figure 1).
Iron (Fe)
Iron is one of the most important minor elements which plays a crucial role in metabolism and central nervous system, the excess intake of Iron leads to tissue damage.17,18 In the present study, the sample contains 1925.6873 ppm of Iron (Table 3 and Figure 1).
Manganese (Mn)
Manganese is the second most important minor element present in plant and animal body which required in various biochemical reactions. In the animal, body Manganese is stored in kidney and liver and it is essential for normal functioning of reproductive and central nervous system.19 Manganese deficiency causes the reproduction failure in male and female. Mn plays a key role in biochemical disorders.20 Some investigations regarding the toxicity of manganese and its translocation from soil to plants confirmed that their importance under low pH and redox potential conditions in the soil. Aluminium hydroxide is used in the treatment of ulcers and kidney. Salts of aluminium used in the synthesis of cosmetics, medicine and control the sweat on the skin. It is one of the trace metals shows less toxicity when takes orally.21 In the present study, the sample contains 98.4683 ppm of Manganese (Table 3 and Figure 1).
Zinc (Zn)
It has an essential role in the processes of genetic expression. It is a vital element having a prominent role in metabolism.22 Zinc is essential for normal development and function of cell mediating innate immunity, neutrophils, and natural killer cells. The concentration of Zinc plays important role in Phagocytises, cytokines production, growth and function of T and B cells, DNA synthesis, RNA transcription and cell activation.23 Zinc is an essential element as it has a relation with the most number of enzymes and participates in enzymatic reactions.24 Zinc is a cofactor in more than 100 enzymatic reactions, an essential component of nuclear DNA binding proteins and serves in genes’ codes for metallothioneins.25 In the present study, the sample contains 10.3987 ppm of Zinc (Table 3 and Figure 1).
Copper (Cu)
The high concentration of copper in the foetal liver is remarkable. Not only is there a massive build-up of liver copper in the normal child during the last three months of pregnancy, but the effect lasts about 4 years, by which time liver copper will have normally reverted to adult levels. This build-up of liver copper ensures adequate supplies for the infant in the first few months. Copper is essential for the growth as well as the health of the animals and plants. Copper deficiency may cause anaemia, bone changes and neutropenia in animals.26 It is an essential element for human. The high liver copper may simply be a reflection of the high demand of the foetus for copper. It must be said, however, that growth rates in the newborn appear to be more closely related to zinc than to copper. The excess intake of copper causes adverse effects such as hypertension, sporadic fever, uraemia’s, coma etc.27 Copper is an essential trace element which participates in many enzymatic reactions. Its most important role has in the redox process. Reactive copper can participate in liver damage directly or indirectly through kupffer cells stimulation.28 In the present study, the sample contains 3.9858 ppm of Copper (Table 3 and Figure 1).
Nickel (Ni)
Nickel may act as a nucleic acid stabilizer as it is present in DNA and RNA. The high concentration of copper in the foetal liver is remarkable. Nickel will enter into the human body mainly through water and food, also living organisms wills mostly exposed to nickel by air. In the present study, the sample contains 4.7803 ppm of Nickel (Table 3 and Fig. 1).
Chromium (Cr)
Chromium is one of the most important trace element required to humans, it plays a vital role in glucose tolerance.29, 30 It is an enzyme activator actively participates in metabolism. If chromium takes in high levels, it causes failure of kidney, liver and lungs, whereas low quantity intake of this may lower the insulin activity. In the present study, the sample contains 10.8653 ppm of Chromium (Table 3 and Fig. 1).
Vanadium (V)
Vanadium is one of the most powerful elements having an important role in metabolism, it lowers plasma cholesterol. The high quantity intake of vanadium leads to severe diseases like gastrointestinal disturbances. It is one of the most important trace elements found in anti-cancer medicinal plants. In the present study, the sample contains 3.7569 ppm of Vanadium (Table 3 and Figure 1).
Conclusions
The present study concludes that the investigation of metals in C. prostrate, the results of this study will be helpful pharmacologist and others to identify the plants and their products useful for research and health. The present investigation deals with trace elements concerning C. prostrate medicinal plant reveals that the elements have significant roles in fighting human ailments and diseases. The study reveals that the presence of various vital trace elements in the medicinal plant is useful for human and animals. Important elements like aluminium, iron, chromium and zinc are present with good values. The toxic metals reported in the present analysis are less and as per the World Health Organization.
Conflict of Interest
Authors declare no conflict of interest.
Funding information
No financial support has been received.
Author’s contribution
Dr. M. Balakrishna: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Dr. P. Seetha Ram: Conceived and designed the experiments; performed the experiments.
Acknowledgements
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references to this manuscript. The authors are also grateful to authors/editors/publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.


References:
-
Dharmasiri MG, Jayakody JRAC, Galhena G, Liyanage SSP, Ratnasooriya WD. Anti-inflammatory and analgesic activities of mature fresh leaves of Vitex negundo. J Ethno Pharmac 2003;87:199-206.
-
Burkill HM. The Useful Plants of West Tropical Africa. Roy Bot Gard Kew 1995;1:58-59.
-
Shirin K, Imad S, Shafiq S, Fatima K. Determination of major and trace elements in the indigenous medicinal plant Withania somnifera and their possible correlation with therapeutic activity. J Saudi Chem Soc 2010;14:97-100.
-
Suresh PP, Ramanaiah M, Ramaraju B. Quantitative determination of essential and trace element content of some medicinal plants by ICP-MS technique. Res J Pharm Tech 2019; 12(4):1595-1600.
-
Raez L, Bumbalova A, Harangozo M, Tolgessy J, Tomecek O. Determination of caesium and selenium in cultivated mushrooms using radionuclide x-ray fluorescence technique. J Radio Anal Nucl Chem 2000;245(3):611-614.
-
Richardson DHS, Shore M, Hartree R, Richardson RM. The use of X-ray fluorescence spectrometry for the analysis of plants, especially lichens employed in biological monolith. Sci Tot Env 1995;176:97-105.
-
Viksna A, Lindgren ES, Standzenieks P. Analysis of pine needles by XRF scanning techniques. X-Ray Spect 2001;30:260-266.
-
World Health Organization. Quality Control Methods for Medicinal Plant Materials, WHO Offset Publication. WHO Geneva, 1998.
-
Frans Kok J, Cornelia VM, Hofman A, Gigsbert B,Voet V. Quality Control Methods for analysis. Epiderm 1988;128:352–359.
-
Unice KU, Monnot AD, Gaffney SH. Inorganic cobalt supplementation: Prediction of cobalt levels in whole blood and urine using a bio kinetic model. Food Chem Toxic 2012; 50:2456–2246.
-
Galanis A, Karapetsas A, Sandaltzopoulos R. Metal-induced carcinogenesis, oxidative stress and hypoxia signalling. Mutat Res 2009;674:31-35.
-
Anke M, Angelow L. Rubidium in the food chain. Fres J Anal Chem 1995;352:236–239.
-
Varo P, Saari E, Paaso A, Koivistoinen P. Strontium in Finnish foods. Int J Vitam Nutr Res 1982;52:342–350.
-
Tayfur M, Unluoglu I, Bener O. Aluminium and health. Food 2002;27(4):306-309.
-
Toxicological profile for aluminium, Agency for Toxic Substances and Disease Registry, Atlanta, USA. 2010.
-
Scientific Opinion on the use of cobalt compounds as additives in animal nutrition. EFSA J 2009;7:1-45.
-
Swanson CA. Iron intake and regulation: implications for iron deficiency and iron overload. Alcohol 2003;30(2):99-102.
-
Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci 2014;19(2):164-174.
-
Fu ZH, Xie MY, Zhang ZM, Guo L. Determination of inorganic elements in Plantago by ICP-AES. Spectr Spe Anal 2004;24(6):737-740.
-
Rehnberg GL, Hein JF, Carter SD, Linko RS, Laskey JW. Chronic ingestion of Mn3O4 by rats: tissue accumulation and distribution of manganese in two generations. J Toxic Envt Heal 1982;9(2):175-188
-
WHO. Trace elements in human nutrition and health. World Health Organization, Geneva. 1996; 343.
-
Mocchegiani E, Giacconi R, Malavolta M. Zinc signaling and subcellular distribution: emerging targets in type 2 diabetes. Trends Mol Med 2008;14(10):419-428.
-
Prasad AS. Zinc. An antioxidant and anti-inflammatory agent: Role of zinc in degenerative disorders of ageing. J Trace Elem Med Bio 2014;28(4):364-371.
-
Cempel M, Nikel G. Nickel: A review of its sources and environmental toxicology. Polish J Env Stud 2006;15(3):375-382.
-
Kamal EAA, Eshteag MM. Influence of end-stage renal disease in alteration of some trace elements in Sudanese patients. Int J Curr Res Rev 2016;8(10):16-19.
-
Krupanidhi S, Sreekumar A, Sanjeevi CB. Copper and biological health. Ind J Med Res 2008;128(4):448-461.
-
Anderson RA. Chromium as an essential nutrient for humans. Reg Toxic Pharm 1997;26(1) 35-41.
-
Sunil G, Shravn Kumar M, Jitendra A, Vishnu Dutt B. A study of trace elements (iron, zink, copper, selenium) in liver cirrhosis patients. Int J Curr Res Rev 2012;4(16):69-75.
-
Geyikli ?, Bay?l S. Chromium associates with insulin sensitivity. Gazi Med J 2008;14:59-63.
-
WHO. Vanadium, in Air Quality Guidelines - Second Edition. WHO Regional Office for Europe; Copenhagen, Denmark: 2000.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License