IJCRR - 8(3), February, 2016
Pages: 23-27
Date of Publication: 11-Feb-2016
Print Article
Download XML Download PDF
SERUM LACTATE DEHYDROGENASE LEVELS IN GASTROINTESTINAL TRACT CARCINOMA PATIENTS BEFORE AND AFTER SURGERY
Author: V. Bhagyalakshmi, N. Sundara Veena, C. H. Ratna Kumar, G. V. Benerji, M. Jaiprakash Babu
Category: Healthcare
Abstract:Background and Objectives: To study the role of Serum Lactate Dehydrogenase (LDH) as a diagnostic parameter in gastrointestinal tract (GIT) carcinoma patients. To compare the level of serum Lactate Dehydrogenase in metastatic and non metastatic Gastrointestinal tract carcinoma patients. To evaluate preoperative and post operative serum Lactate Dehydrogenase level in Gastrointestinal tract carcinoma patients. Materials and Methods: Studied 20 patients of Gastrointestinal tract carcinoma admitted in surgical ward were selected for study group. Out of 20 patients, 15 patients were Gastrointestinal tract carcinoma without metastasis. 5 patients were Gastrointestinal tract carcinoma with metastasis. 20 healthy individuals were included in the control group. The correlation of serum Lactate Dehydrogenase levels in pre operative and post operative Gastrointestinal tract carcinoma patients were studied. Results: Serum Lactate Dehydrogenase levels were elevated in pre operative GIT carcinoma patients with and without metastasis. Serum Lactate Dehydrogenase levels were decreased in post operative patients of Gastrointestinal tract carcinoma without metastasis but elevated in postoperative patients with metastasis. Conclusion: The study showed serum Lactate Dehydrogenase as an independent diagnostic and prognostic bio marker.
Keywords: GIT carcinoma, Lactate Dehydrogenase, Metastasis
Full Text:
INTRODUCTION
Lactate Dehydrogenase (LDH) is an enzyme universally distributed in various tissues of the body. LDH is a cytoplasmic enzyme reversibly catalyses the conversion of pyruvate to lactate. Pyruvate+NADH+H+ L lactate+NAD+ Recently metabolic reprogramming has been recognized as a hall mark of cancer. Tumor cells produce a substantial amount of their energy through glycolysis. Cancer cells utilize glycolysis for energy production under normoxic conditions. This allows cancer cells to sustain higher proliferation rates. Due to rapid tumor cells divided, high metabolic demands, tumor avascular area formation, hypoxia is a characteristic property in solid tumor cells, in turn convert majority of their glucose stores into lactate. Gastro intestinal carcinoma is one of the most common cause leading to death in developed as well as developing countries. The more common gastrointestinal carcinomas are of stomach, colorectal, esophageal, pancreas and biliary tract. Gastric cancer is the fourth most commonly diagnosed malignancy and the second leading cause of cancer death in worldwide [1]. The main problem of GIT tract malignancies is late detection of disease as the symptoms appear in late stage. The increasing rate of mortality in case of GIT carcinoma is also due to poor screening as well as negligence by the patient itself specially in developing countries. So the main challenge is to detect the cancer in early stage so that curative surgery can be done which has better prognosis.
A biochemical marker which acts as an indicator of the disease at the beginning can be really be helpful in providing better management. Lactate Dehydrogenase enzyme estimation provides a better prognostic indicator[2]. Biochemical screening can be done by estimating serum lactate dehydrogenase either pre operatively for diagnosis or post operatively for prognosis. Serum lactate dehydrogenase is estimated to evaluate its diagnostic as well as prognostic implication in established GIT carcinoma cases[3]. Serum LDH has been found elevated in serum of GIT carcinoma cases. The degree of elevation of serum LDH closely reflects the clinical status of the patient. In case of distant metastasis the level of serum LDH is found to be significantly high. This study showed Serum LDH as tumor marker of GIT tract carcinoma. As per cost wise this investigation is highly suitable, cheaper, accurate, feasibility as compared to any invasive procedure and is found to be higher in GIT carcinoma as well as higher in GIT carcinoma with metastasis.
MATERIALS AND METHODS This is a prospective randomized study done for one year at Department of Biochemistry and the samples were collected from Department of General Surgery, Rangaraya Medical College, Kakinada. Samples were collected from two groups. They were: Study Group: 20 patients were selected with various types of GIT carcinomas from General Surgery wards of Government General Hospital, Kakinada. Control Group: 20 healthy individuals were selected. Persons who did not have a medical history of Blood, skeletal muscle, cardiac, hepatic or renal diseases and any type of cancer previously which are associated with increased serum LDH levels. Consent was taken from the patients and Ethical clearance has approved.
The medical report was kept confidential. Detailed history and thorough clinical examination was done. Special investigations like ultrasound, endoscopy and radiological investigations and other investigations as the case demanded. After confirmation of GIT carcinomas by the investigations. Blood Samples were collected preoperatively, hemolysis was avoided as it increases serum LDH levels. This was followed by laparotomy with definite surgery to patients with GIT carcinoma, excised tissue was sent for histopathology examination for confirmation of Carcinoma.
Out of 20 patients -15 patients were GIT carcinomas without metastasis 05 patients were GIT carcinomas with metastasis. Confirmation of pathological diagnosis of GIT carcinoma done by pathology department of Andhra Medical College, Visakhapatnam. Blood Samples were also collected from study group postoperatively on 7th day as routine follow up of cases after surgery for prognostic purpose and also from control group to estimate serum LDH levels. Methodology: Measurement of Serum LDH levels in both control and study group by UV kinetic method. Lactate dehydrogenase catalyses the conversion of pyruvate to lactate and the reduced coenzyme NADH to NAD+ . LDH activity in the sample is directly proportional to the rate of decrease in the absorbance of NAD+ at 340nm. Pyruvate + NADH + H+ ------LDH Lactate + NAD+ Statistical analysis: Statistical analysis has done by calculating p value using graphpad software.
RESULTS Among total 20 study group, 15 patients diagnosed as GIT carcinoma without metastasis and 5 patients diagnosed as GIT carcinoma with metastasis. Various type of Carcinomas were evaluated. Those GIT carcinomas were depicted in Table no:1. In one year study Carcinoma of stomach was most commonly diagnosed among various GIT carcinomas.

Among study group patients were in the age group of 35-75 years. Age and sex wise distribution were tabulated in Table No:2

DISCUSSION Tumor cells has capacity to proliferate infinitely, which causes alteration in the metabolism of cells in turn there is a change in the levels of various factors like hormones, enzymes. Exploring these objective indicators is extremely important for clinical practice [4,5]. Serum LDH is a non-functional enzyme and the level is very low in growing periods. They appear in plasma only after destruction of erythrocytes and other cells. The pattern of rise of this intracellular enzyme vary according to involvement of specific organ and its rise in blood is helpful in the diagnosis of specific disease. Several independent studies have shown that increased serum LDH levels or LDH-5 expression (as detected by immunohistochemistry) predicted poor prognosis and high metastasis risk in a spectrum of neoplastic diseases, including breast cancer, colorectal cancer, non–small cell lung cancer, endometrial cancer, and gastric cancer[6,7].
In malignancy the newer cells are being generated and the large number of malignant cells are destroyed by tumor necrosis factors and others. As discussed earlier this process leads to rise of various intracellular enzymes level in blood. In this study only SLDH was taken on the same lines that the cell destruction might result in increased level of it, especially in GIT Carcinoma. LDH was recently recommended as an objective indicator of tumor prognosis[8,9]. As per this study, in the study group 20 cases of various types of GIT Carcinoma were selected in which carcinoma esophagus consisted 25%, carcinoma stomach 30%, Carcinoma colon 20% and carcinoma rectum 25%. Gastric cancer is the fourth most commonly diagnosed malignancy and the second leading cause of cancer death in worldwide. Although the prognosis of gastric cancer has improved in the recent few decades, the overall 5-year survival rate is still poor [1,10]. The main causes of death in gastric cancer patients are recurrence and metastasis.
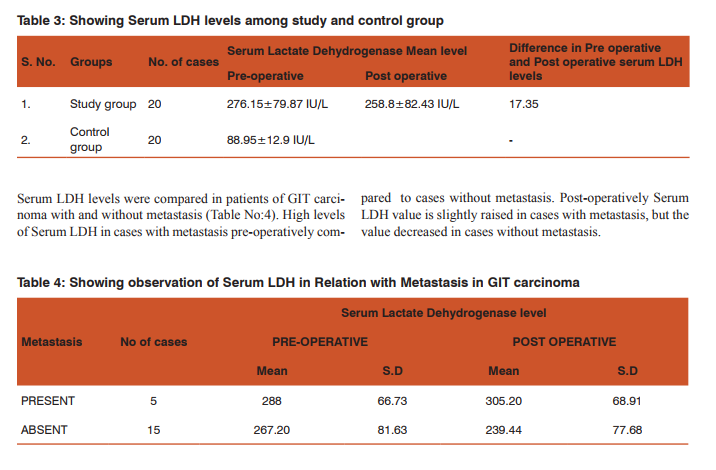
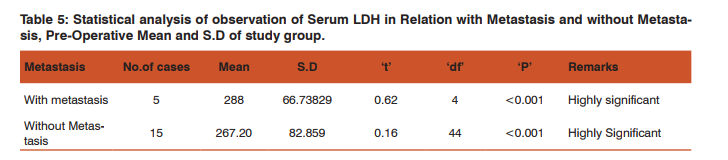
There is much difference in serum LDH levels in between preoperative and postoperative estimation about 17.35IU/L. High levels of Serum LDH in cases with metastasis preoperatively compared to cases without metastasis. Post-operatively Serum LDH value is slightly raised in cases with metastasis (0.25%), but the value decreased in cases without metastasis. Guo Li et al[11] reported that decrease in serum LDH value were 74.9% during therapy. There was no difference in survival between the patients with decreased serum enzyme levels and those without decreased levels. Lee H et al [12] observed that among patients with advanced gastric cancer, high serum LDH level was related to better response to chemotherapy but shorter survival duration. The normalization of elevated serum LDH level after chemotherapy was related to good response to treatment.
CONCLUSION We conclude that from this study, elevated SLDH levels in this study showed as independent diagnostic marker in preoperative GIT carcinoma patients with and without metastasis. Decreased SLDH levels in postoperative GIT carcinoma patients without metastasis showed as a prognostic marker. Elevated SLDH levels in GIT carcinoma patients with metastasis showed as a poor prognostic marker.
ACKNOWLEDGEMENTS We are thankful to Biochemistry technical personnel for aiding us in doing the present study. We also grateful to authors/ editors of all publishers and books in the references cited.
References:
1. Atrkar-Roushan Z, Kazemnejad A, Mansour-Ghanaei F, and Zayeri F. Trend analysis of gastrointestinal cancer incidences in guilan province: comparing rates over 15 years. Asian Pacific Journal of Cancer Prevention 2013;14(12):7587–7593.
2. Russell J Erickson. Lactic dehydrogenase activity of effusion fluids as an aid to differentiate diagnosis. JAMA 1961 Jun3;176(9):794-796.
3. Morton K Schwartz. Enzymes as prognostic markers and therapeutic indicators in patients with cancer. clinica chimica acta 1992 Mar13;206: 77-82.
4. Bidart JM, Thuillier F, Augereau C, Chalas J, Daver A, Jacob N, Labrousse F, Voitot H. Kinetics of serum tumor marker concentrations and usefulness in clinical monitoring. Clin Chem. 1999 Oct; 45(10):1695-707.
5. Patel PS, Rawal GN, Balar DB. Combined use of serum enzyme levels as tumor markers in cervical carcinoma patients. Tumour Biol. 1994; 15(1):45-51.
6. Kolev Y, Uetake H, Takagi Y, Sugihara K. Lactate dehydrogenase-5 (LDH-5) expression in human gastric cancer: association with hypoxia-inducible factor (HIF-1alpha) pathway, angiogenic factors production and poor prognosis. Ann Surg Oncol. 2008 Aug;15(8):2336-44.
7. Koukourakis MI, Giatromanolaki A, Sivridis E, Bougioukas G, Didilis V, Gatter KC, Harris AL. Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis, Tumour and Angiogenesis Research Group. Br J Cancer. 2003 Sep 1; 89(5):877-85.
8. Füssenich LM, Desar IM, Peters ME, Teerenstra S, van der Graaf WT, Timmer-Bonte JN, van Herpen CM. A new, simple and objective prognostic score for phase I cancer patients. Eur J Cancer 2011 May; 47(8):1152-60.
9. Arkenau HT, Barriuso J, Olmos D, Ang JE, de Bono J, Judson I, Kaye S Prospective validation of a prognostic score to improve patient selection for oncology phase I trials.. J Clin Oncol. 2009 Jun 1; 27(16):2692-6.
10. Kunz PL, Gubens M, Fisher GA, Ford JM, Lichtensztajn DY and Clarke CA. Long-term survivors of gastric cancer: a California population-based study. Journal of Clinical Oncology 2012; 30(28):3507–3515.
11. Guo Li, Jin Gao, Ya-Lan Tao, Bing-Qing Xu, Zi-Wei Tu, ZhiGang Liu et al. Increased pretreatment levels of serum LDH and ALP as poor prognostic factors for nasopharyngeal carcinoma. J Cancer. 2012 Apr; 31(4): 197–206.
12. Lee H, Yuh Y, Kim S. Serum lactate dehydrogenase (LDH) level as a prognostic factor for the patients with advanced gastric cancer Sanggyepaik Hospital, Seoul, Republic of Korea. J Clin Oncol 2009;27.
13. Kemeny N et al. Prognostic factors in advanced colorectal carcinoma, Importance of lactic dehydrogenase level, performance status and white blood cell count. AM J Med 1983.
14. Michael I Koukourakis et al. Prognostic and predictive role of LDH5 expression in colorectal cancer patients treated with PTK 787/2K 222584 anti angiogenic therapy. Clin Cancer Res 2011 Jul 15;17:4892-4900.
15. Najib R et al. P-O 217-Prognostic factors in colorectal cancer, serum LDH Levels predict survival in metastatic disease. Annals of oncology 2014;25(2).
16. Ghool AM et al. A comparative study of LDH and PchE in sera of cancer patients , a preliminary report . Indian J .cancer 1980;17:31-3.
17. Cornelia Hoch ligeti et al. Effect of malignant growth on the organ lactic dehydrogenase in the human. cancer 2006 Jun 23;18(9).
18. Nyandieka HS. Influence of therapeutic radiation on serum enzyme levels in patients with cancer of thoracic esophagus . Indian J. Med. Res. 1984 jun;794-800.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License