IJCRR - 13(4), February, 2021
Pages: 176-179
Date of Publication: 16-Feb-2021
Print Article
Download XML Download PDF
Antimicrobial Activity of Alkaloids from Withania Somnifera and Clitoria Ternatea
Author: Thamilarasu R, Arun Kumar M, Vignesh M, Pavithra MKS
Category: Healthcare
Abstract:Introduction: Withania somnifera and Clitoria ternatea are commonly used herbs for the preparation of Ayurvedic products to treat most health problems. Objective: The present study was carried out to determine the Minimum Inhibitory Concentration (MIC) against microbes in stagnated rainwater. Methanolic extract of Withania somnifera root and Clitoria ternatea root were prepared and subjected to identification of the presence of alkaloids. Methods: Thin Layer Chromatography (TLC) method and the concentration was measured by UV spectrometry. Solvent extraction was carried out by maceration, orbital shaker and microwave oven methods. The extract was concentrated by the distillation process. The extract of W.somnifera collected from room temperature and the methanolic extract of C.ternatea was subjected to TLC method with the methanol as mobile phase. The compounds separated in the TLC plate was scraped off and subjected to UV spectrophotometry to identify the concentration of alkaloids present in the extract of both W.somnifera and C.ternatea. The extracts were tested for the anti-bacterial and anti-fungal activity. Results: The results found that the extraction of alkaloid compounds from W.somnifera can be enhanced by the microwaveassisted extraction and there was no change in the amount of extraction of alkaloids in C.ternatea. Finally, both the extract was subjected to anti-bacterial and anti-fungal activity. Different extracts from a single plant extracted by different extraction process show the different antimicrobial effect on microbes on stagnant rainwater. The type of extraction affects the anti-microbial effect of the extracts.
Keywords: Withania somnifera root, Clitoria ternatea, Distillation, TLC, Anti-microbial
Full Text:
Introduction
Withania somnifera extracts possess multiple biological activities like cardio-protective, neuro-protective, anticancer, and anti-inflammatory.1-4 Withanolides and phenolic compound aids in the medicinal properties of this plant. Withanolides A exhibits anticancer and anti-inflammatory activities.5
Clitoria ternatea used for the treatment of snakebite and scorpion string, skin disease, asthma and pulmonary tuberculosis.6,7 The active chemical constituents are tannins, resins, taraxerol, flavonoids, saponins and starch.8 The plant extract shows strong anti-microbialeffect.9 This experiment was carried to determine comparative analysis between these plants and for the isolation of alkaloids from the W.somnifera extract. The experiment was carried out to find the antimicrobial effects of both the plant extract extracted by various extraction method against the microbes in stagnant rainwater.1,10
Methodology
Sample collection
Both plant samples were collected from the nearby area of Sathyamangalam in Erode district. The roots were separated from it and dry it in sunlight until all the moisture removed from the root. Later the dried root part was subjected to grinding to make it powder-like substance and store it.11
Extraction from root
Three types of extraction for both samples were carried out with the methanol as solvent. The powder along with the solvents was placed in Microwave oven, Room temperature, Orbital shaker. In the microwave oven, 100 g of root powder were dissolved in 150 ml methanol and subjected to 180°C for 60 sec. The extract was filtered with Whatman filter paper and this above method was repeated for the collection of 250 ml extract. In-Room temperature, the same amount of powder and solvent were taken as the microwave condition. The mixture was allowed to stands for 4–5 hrs and the extract was filtered. In Orbital shaker, the mixture was kept in 80-95 rpm and the extract was filtered from it. All the extracts were concentrated using a rotary evaporator.12
Spectral analysis
The spectral analysis was performed using UV-Vis spectrophotometer. The absorbance measured under the UV light at 210 – 700 nm range using a UV visible spectrometer.11
Thin Layer Chromatography Studies
Thin layer Chromatography method is used to separate the non-volatile component. Pre-coated and pre-activated TLC Silica gel 60 F254 plates used in the study was procured from Merck Specialties Private Limited. The samples were loaded on the TLC plate 0.50 cm above its bottom using capillary glass tubes. After the application of the sample, the plates were kept insolvent saturated TLC glass chamber for the elution of the molecules. The solvent system used for fingerprinting analysis is Chloroform: Methanol (15:1). Dragendroff's reagent is sprayed over the plate. Observe the Orange-brown spot in the plate, spray the sodium nitrate reagent if the intensity of the colour is faded. Finally, the dark brown colour was obtained.10
Media Preparation
In 250 ml conical flask, take 150 ml distilled water and add 4.2 g of nutrient agar and 2 g agar was added for solidification of media, heat to dissolve it and autoclaved at 121°C, the pressure of 15psi for 15min.13
Antimicrobial studies
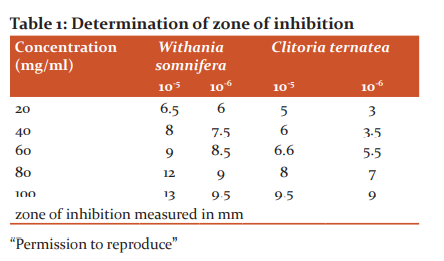
Various concentrations of the extracts were prepared in sterile water and the resultant extracts were used to determine the antimicrobial activity through well diffusion method. 15 ml of stagnated water was collected in a beaker. After media preparation, 10-5 and 10-6 times diluted stagnant water was swabbed over the media in 4 Petri plates to test the inhibition activity. 5 wells were punched using a sterile syringe. In each well, different concentration of extracts of both the extracts in the respective Petri plates was added 20 µl, 40 µl, 60 µl, 80 µl and 100 µl respectively. The plates were incubated at 37?C for 7 days. The radius for the zone of inhibition was measured in millimetres and recorded against the corresponding concentration. Experiments were carried out with three replicates per treatment. The respective inhibition zone was measured in each of the plates. The experiments were carried out in triplicates and the mean values are considered along with the standard deviation.6,7,12
Gram Staining
Gram staining was done to identify the type of microorganism present in the stagnant water. Crystal violet was added to the heat fixed organism in the slides. After washing in the flow of sterile water, few drops of iodine were added. After 1 min, the iodine was removed by washing with water. Then the slides were washed with the decolourizer subsequent washing with water. Finally, safranin was added and washed with water. Then the slides were observed under the microscope to identify the presence of either gram-negative or gram-positive organisms.12 The colonies were isolated and subjected to sequencing for species identification.
Results and Discussion
Dragendroff’s reagent test
Dragendroff’s reagent test was performed with the 3 root extracts to confirm the presence of alkaloids by observing the colour change to orange. The presence of alkaloids (Figure 1) was confirmed by this test.
UV Spectrometer
Estimation of biomolecules by UV spectrophotometer was carried out to estimate the presence of alkaloids at the λmax using Double beam, UV-VIS (Make: Perkin Elmer & Model: Lambda 35) Spectrophotometer. The result (Figure 2) showed that the peak value to be 1.928 which confirm the presence of Withaferin A in microwave-assisted extraction. The results (Figure 3) showed that the peak value to be 2.321 which confirm the presence of alkaloid in C. ternatea extract prepared using microwave-assisted extraction.
Thin-layer chromatography
Thin-layer chromatography aids in better identification of therapeutically active compounds. The TLC profiling of the leaf extracts shows the presence of diverse metabolites such as alkaloids, flavonoids, phenols, and tannins. The chromatogram depicts the differences in the concentration of biomolecules possessed by the plant by distinguishing the intensity of the secondary metabolites. The Rf values of the molecules provide a shred of evidence about the polarity that shall aid in selecting the particular solvent system for extended studies on the characterization of metabolites using spectroscopic and chromatographic techniques. Low polarity molecules show high Rf value in a less polar solvent system, whereas high polarity molecules have a low Rf value. The results of TLC confirm the presence of alkaloids at the RF 0.66 (Figure 4) in W. somnifera and Rf 0.53 (Fig 4) in C. ternatea.
Antimicrobial Assay
The stagnated water was spread over the media and (Figure 5). The zone of inhibition was measured in each well and the results are tabulated.
Gram Staining
The colonies on the plate were purified, stained and observed under the laboratory microscope. The presence of both gram-positive and gram-negative microorganisms in the water sample was confirmed. The antimicrobial activity of the plant extracts with the pure cultures (Figure 6) was determined. The zone of inhibition was measured (Table 1). It was thus validated that the plant extracts possess antimicrobial activity towards both gram-positive and gram-negative bacteria.
Conclusion
The different parameters of extraction including ethanolic extraction at room temperature, microwave-assisted extraction, orbital shaker assisted extraction, screened using two-level factorial design. Each factor was evaluated to find out the most significant and contributing factor in achieving high extraction yield and total alkaloid content of ashwagandha extract. Results indicated that ethanolic extract assisted by microwave was the most significant and contributing factor affecting the results of ashwagandha extracts, while extraction room temperature and extract assisted by orbital shaking exhibited the least significance and contribution. The factor levels for screening purpose was selected based on OFAT experimental design. The results also found that the extraction of bioactive compounds from ashwagandha can be enhanced by enhancing the microwave-assisted extraction parameter. Different types of microorganisms both gram-positive and gram-negative microbes are present in the stagnant rainwater. The above result shows that these both extracts act growth-inhibiting agents indicating that these compounds can be a microbial inhibiting factor. The pure form of this compound is extracted by the TLC methods. The possible interaction between the drug and excipient will be studied by FTIR spectroscopy to show the interaction between the drug and polymer. The extract can be used in the form of either powder or tonic for many medicinal purposes mentioned above. Preparation, attractiveness and the drug release characteristics, hard candy lozenges are ideal and attractive alternatives for drug delivery from Lidocaine lozenges for its Local anaesthetic action.
Source of Funding
The work is self-supported
Conflict of Interest: None
Conflicts of interest
There are no conflicts of interest.
Acknowledgement:
The authors are thankful to The Chairman, Trustee, and The Principal of Bannari Amman Institute of Technology, Sathyamangalam, for constant support and continuous encouragement.
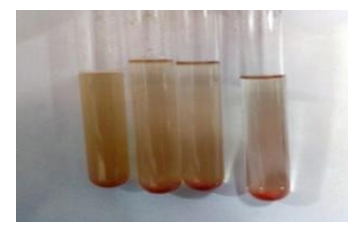
Figure 1. Dragendroff’s reagent test for each of three forms of extraction. The colour change to reddish orange supports the presence of alkaloids in the extract.
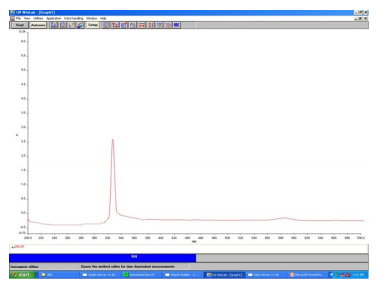
Figure 2. UV spectrum for withaferin A for microwave assisted extraction. The λmax at 330 nm confirms the presence of Withaferin A with absorbance 1.928 Å.
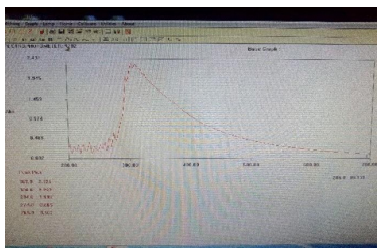
Figure 3. UV spectrum for Clitoria ternatea extract. The λmax at 290 nm confirms the presence of indole alkaloid in C.ternatea extract with an absorbance of 2.321 Å.

Figure 4: TLC for microwave assisted extraction Withania somnifera and Clitoria ternatea.
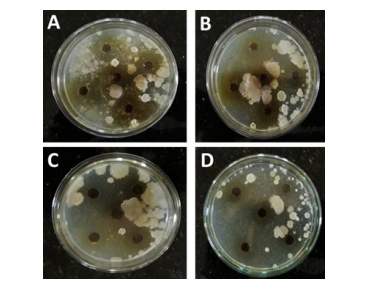
Figure 5: Antimicrobial activity of Withania somnifera and Clitoria ternatea at two different dilutions supporting. The absence of microbial growth on and near the wells supports the potential of plant extracts in inhibiting the growth of microorganisms present in stagnated water. A: Withania somnifera at 10-5 dilution; B: Withania somnifera at 10-6 dilution; C: Clitoria ternatea at 10-5 dilution; D: Clitoria ternatea at 10-6 dilution
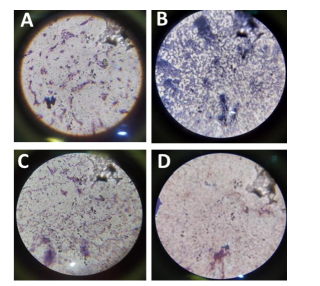
Figure 6: Results of Staining using Grams reagent revealing the presence of both gram positive and negative strains. A: Gram Negative organism from 10-5 dilution; B: Gram Positive organism from 10-5 dilution; C: Gram Positive and Negative organism from 10-5 dilution; D: Gram Negative organism from 10-6 dilution
References:
1. Barik DP, Naik SK, Mudgal A, Chand PK. Rapid plant regeneration through in vitro axillary shoot proliferation of butterfly pea (Clitoria ternatea L.) a twinning legume, In vitro. Cell Dev Bio-Plan 2007;42:144-148.
2. Dhar N, Razdan S, Rana S, Bhat WW, Vishwakarma R and Lattoo SK. A decade of molecular understanding of withanolide biosynthesis and in vitro studies in Withania somnifera (L.) Dunal: Prospects and Perspectives for Pathway Engineering. Front Plant Sci 2015;1026:1031.
3. Mohanty I, Gupta SK, Talwar KK. Cardioprotection from ischemia and reperfusion injury by Withania somnifera: A hemodynamic, biochemical and histopathological assessment. Mol Cell Biochem 2004; 260:39–47.
4. Chandra P, Kannujia R, Saxena A. Quantitative determination of multi markers in five varieties of Withania somnifera using ultra-high-performance liquid chromatography with hybrid triple quadrupole linear ion trap mass spectrometer combined with multivariate analysis: Application to pharmaceutical dosage forms. J Pharm Biomed Anal 2016; 129:419-426.
5. Oguis GK, Gilding EK, Jackson MA, Craik DJ. Butterfly Pea (Clitoria ternatea), a cyclotide-bearing plant with applications in agriculture and medicine. Front Plant Sci 2019; 610:645.
6. Alok S, Gupta N, Kumar A and Malik A. An update on Ayurvedic herb Vishnukanta (Clitoria ternatea Linn.): A review. Int J Life Sci Rev 2015;1(1):1-9.
7. Kumar V, Dey A, Hadimani MB, Marcovic T, Emerald M. Chemistry and pharmacology of Withania somnifera: An update. Tang Hum Med 2015;5(1):23-26.
8. Kannan KP, Poorani T, Venupriya V, Sathya R, Sivapriya V, Senthamarai M, Pavithra MKS, Madhankumar D. Comparative studies on the phytoconstituents, antibacterial and pesticidal activities of blue and white varieties of Clitoria ternatea Linn. Acta Biomed Sci 2016;3(4):218-213.
9. Sethiya NK, Trivedi A, Patel MB, Mishra SH. Comparative pharmacognostic investigation on four ethnobotanicals traditionally used as Shankhpushpi in India. J Adv Pharm Tech Res 2010;1(4):388-395.
10. Petruczynik A, Waksmundzka-Hajnos M, Michniowski T. Thin-layer chromatography of alkaloids on cyanopropyl bonded stationary phases, Part I. J Chromatogr Sci 2007; 45(7):447-454.
11. Dhruv KS, Bhavana S, Archana S. Spectrophotometric determination of rauwolfia alkaloids: estimation of reserpine in pharmaceuticals. Anal Sci 2004;20(3):571-573
12. Coico R. Gram staining. Current protocols in microbiology, 2006 (1): A-3C.
13. Rios JL Recio MC. Medicinal plants and antimicrobial activity. J Ethnopharmcol 2005;100(1-2):80-84.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License