IJCRR - 13(4), February, 2021
Pages: 76-82
Date of Publication: 16-Feb-2021
Print Article
Download XML Download PDF
Screening and Production of Lovastatin Producing Endophytic Fungus from Phyllanthus reticulatus using Oyster Mushroom Extract
Author: Senthamarai Manogaran, Kovilloo Packiam Kannan, Kowsalya Rathinasamy, Harish Ranganathan, Govardthni Gurusamy
Category: Healthcare
Abstract:Introduction: Endophytic fungi are most commonly found in plants. They are present inside the plants and supporting plants to survive and produce many bioactive metabolites. Lovastatin is one of the secondary metabolites produced by the endophytic fungi. It is used in the treatment of high blood cholesterol and reduces the risk of cardiovascular diseases. Objective: Screening and Production of Lovastatin Producing Endophytic Fungus from Phyllanthus reticulatus using Oyster Mushroom Extract. Methods: In our study, we have isolated endophytic fungi from the leaves and stem of phyllanthus reticulatus. Biodiversity of the endophytic fungi was calculated. Neurospora crassa bioassay was performed to identify the best producer of lovastatin. Their Lovastatin production potential was investigated with two different mediums such as potato dextrose and the mushroom extract. After completion of the fermentation process, the broth was extracted by a simple distillation method and then analyzed for the presence of Lovastatin using UV-Visible spectrophotometer and HPLC. Results: One of the positive and dominant endophytic fungi, Curvularia sp was isolated and chosen for this study. Its Lovastatin producing potential in PDA and mushroom extract medium was investigated and confirmed. Conclusion: Curvularia sp was identified as a better Lovastatin producer when compared to other endophytic fungi isolated from Phyllanthus reticulatus. From the results of the UV and HPLC, the presence of lovastatin is confirmed.
Keywords: Endophytic fungi, Lovastatin, Bioassay, UV Spectrometry, High-performance liquid chromatography
Full Text:
INTRODUCTION
Endophytic fungi are the probable alternative sources for the development of novel biotechnological products like antibiotics, antimycotics, immunosuppressants and anti-cancer compounds. Such microorganisms live in the plant's internal tissue which supports the plant cells growth and metabolism. Fungal endophytes have been recognized as an excellent source for new compounds of enormous value in agriculture, industry and medicine.1,2 They were the potential sources for the discovery of new and useful compounds or new platforms for the organic synthesis of such compounds for human benefit. Unlike organic synthetics, there is a clear need for endophytic sources of antimicrobials for their biological and chemical safety effects due to health and environmental problems.1,3,4
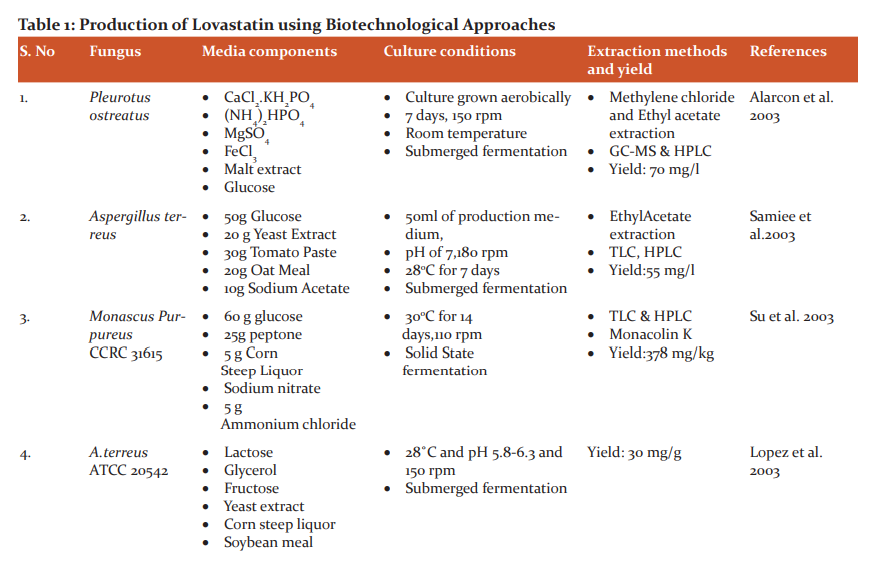
Lovastatin is one of such secondary metabolites having huge medicinal properties and also naturally produced by certain higher fungi, such as Pleurotus ostreatus (oyster mushroom) and closely related Pleurotus sp.5 Chemical synthesis of Lovastatin strictly involves synthetic methods. It was the first statin drug which was patented5 and approved by the United States Food and Drug Administration (FDA) in 1987.6-9 The cost of production is too high for commercialization purposes and other commercially viable chemical processes only yield poor quality production and involve complicated production steps.10-13 Hence, biotechnology has opened a new avenue through simple biotechnological methods to produce high quality and cost-effective Lovastatin and it is summarized in Table 1.
Phyllanthus reticulatus belongs to the family of Euphorbiaceae, usually a dense deciduous shrub which contains lots of medicinal properties. The different parts of this plant such as leaves, dried bark, fruits, aerial parts are to treat various diseases. In the pharmacological view, it has various activities like antidiabetic, antispasmodic, hypocholesterolemic, antimicrobial, cytotoxic, hepatoprotective anti-hyperglycemic, etc.14-19
MATERIALS AND METHODOLOGY
Collection of Plant
Healthy leaf, stem and root of Phyllanthus reticulates (Poir) was collected from the Theravada regions of Sathyamangalam Taluk in a sterile ziplock bag and processed within 24 hours at the Endophytic Fungal Metabolite Research Laboratory, Department of Biotechnology, Bannari Amman Institute of Technology. 20,21
Isolation and Identification of endophytic fungi
All the plant specimens were washed thoroughly with sterile water and endophytic fungi were isolated. 22,23 The surface sterile leaves, stem and petiole were dissected under sterilized conditions into small segments of 0.5 cm x 0.5 cm using a sterile scalpel and dried in blotting paper. They were placed equidistantly (10 segments per plate per specimen) on the freshly prepared Potato Dextrose Agar plates (PDA) amended with Chloramphenicol (50 μgmL-1, Sigma Aldrich) and streptomycin sulphate (250 μgmL-1 Sigma Aldrich) and incubated at 25°C±1°C.24,25
Identification of Endophytic fungi was carried out using microscopic and morphological characteristics. Isolated pure endophytic fungal cultures were stained using lactophenol cotton blue staining method and viewed under the microscope and were shown in Figures 2,3 and 4.
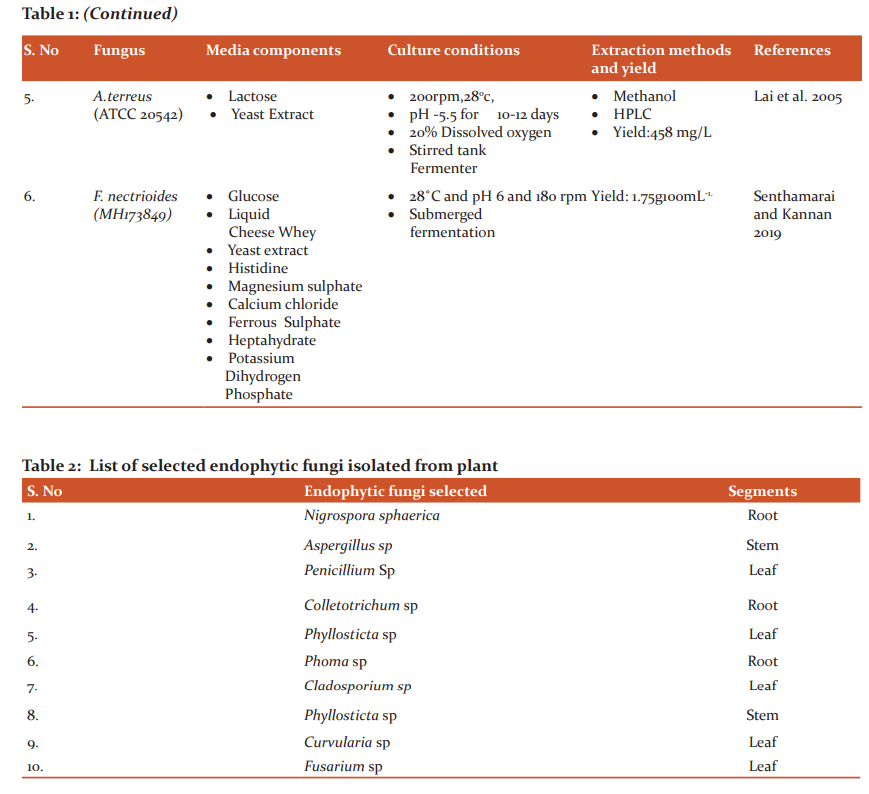
Among the group of endophytic fungi isolated from the plant, best fungal isolate (Table 2) was chosen based on the highest colonization frequency and zone of inhibition in a bioassay.
Biostatistics for Species Diversity
Colonization Frequency Percentage (CF %)
The density of colonization of single endophytes species was calculated and was equal to the number of colonized segments divided by the total number of segments observed x 100.
Endophytic Infection Rate (EIR %)
Endophytic Infection Rate was determined as the number of infected plant segments divided by the total number of plant segments screened x 100.26-30
Screening by Neurospora crassa Bioassay
This screening was based upon the use of biological responses as a detection system for biologically active substances. Neurospora crassa (MTCC 790) was sensitive to β hydroxyl acid of Lovastatin 19 and hence, Lovastatin producing isolates create a zone of inhibition by suppressing the growth of this organism. Thus, using this principle, selected 10 endophytic fungal species were screened to identify their Lovastatin producing capabilities.26,27
Batch fermentation
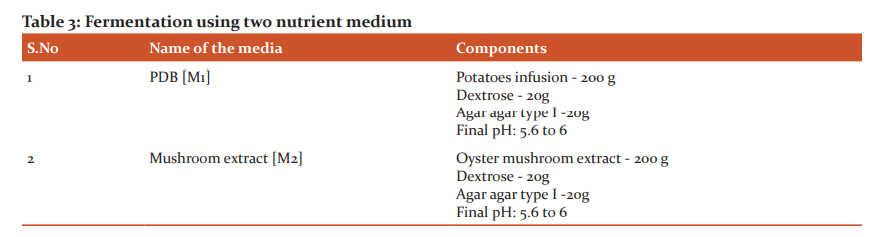
Highly positive endophytic fungi obtained through screening was chosen for the further fermentation process. Hence, Fully grown mycelial culture (1cm x 1cm) of Curvularia sp from the agar plate was inoculated in 100 mL of two different media M1 and M2 (Table 3) at pH 6 in triplicates under sterile condition. All the flasks were incubated in a shaker incubator at 28°C and 180 rpm for 10 days.6,14,16
Extraction and confirmation of Lovastatin production
After 8 days of fermentation, the culture broth was separated by sterilized filter cloth. The culture filtrate was adjusted from pH 6 to pH 2 and was kept in a rotary shaker with an equal volume of ethyl acetate at 100 rpm for 2hrs at room temperature. After the extraction process, the broth was centrifuged at 1500 rpm for 20 min and filtered using Whatman filter paper No.1 to separate the biomass and filtrate. The filtrate was concentrated to 20 ml using rotary evaporator .23,24,25
The presence of Lovastatin in the fermentation broth was confirmed at the maximum absorbance by UV spectrophotometry at 238nm. Further, the sample was analyzed using HPLC with a C18 column as a stationary phase and acetonitrile and water (65:35 v/v) as mobile phase.2
RESULTS
Biostatistics for Species Diversity
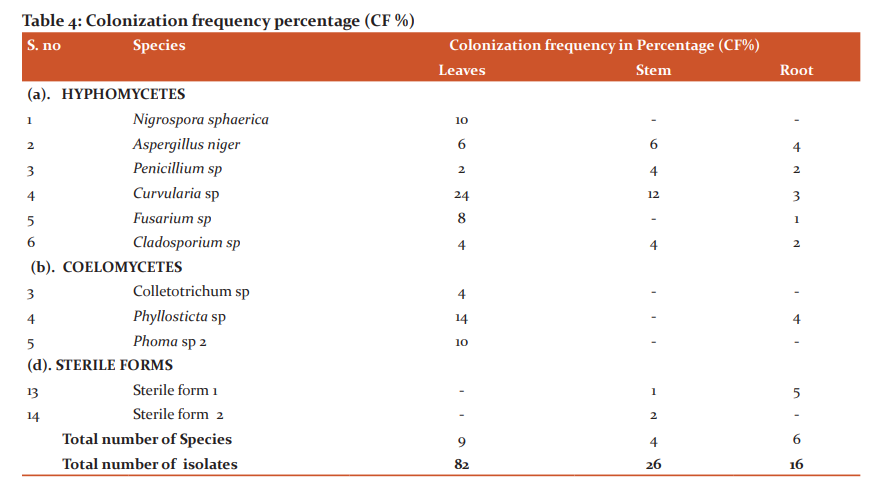
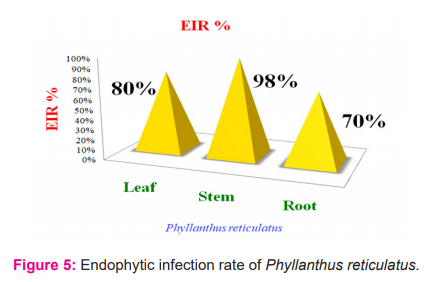
The colonization frequency percentage of the endophytic fungal species were shown in table 4 and the endophytic fungal infection rate percentage of Phyllanthus reticulatus was shown in figure 5.
Screening by Neurospora crassa Bioassay
The zone of inhibition formed by the endophytic fungi against Neurospora crassa is shown in table 5. Among these, the Curvularia sp shows higher zones of inhibition up to 0.6cm when compared to the other organisms. Further studies were carried out using the Curvularia sp.
Batch fermentation
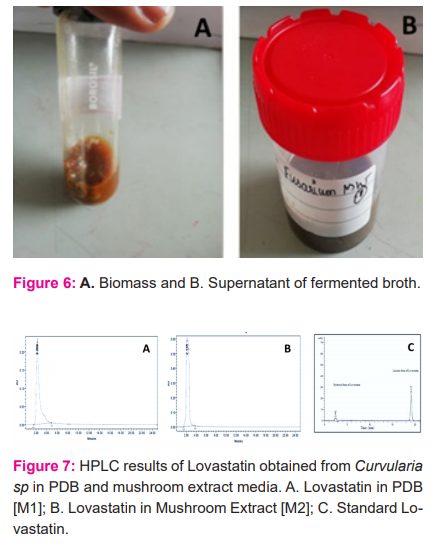
Fermentation of Curvularia was carried out in both PDB and mushroom extract medium. Every 2 days, biomass was collected, filtered using pre-weighed sterilized filter cloth and dried at 60 ?C. Maximum OD of 0.826 in mushroom medium and 0.878 in PDB medium were observed at 238nm under UV spectrophotometer. The HPLC results of both the medium show that the peaks are closer enough to the standard lovastatin. It confirms the lovastatin production by Curvularia sp. The fermented biomass samples were shown in figure 6 and the HPLC results were shown in figure 7.
DISCUSSION
This study revealed that the endophytic infection rate was high in the stem segment of the selected plant and the Curvularia sp were more abundant in the leaves of the selected plant in terms of colonization frequency compared to other endophytic fungi isolated from the same plant.28,29 This may be due to the environmental suitability and interaction of Curvularia sp within the plant. This fungus has also shown a good inhibitory effect against Neurospora crassa which is one of the confirmatory tests for the production of lovastatin. Thus, this dominant fungus was chosen for the investigation of lovastatin production in a shaker flask level. The production of lovastatin by Curvularia sp in the mushroom extract and PDB medium was confirmed by UV and the HPLC. The Rf value of lovastatin in the mushroom extract was 2.135 which is close to the hydroxyl form of standard lovastatin.30-32
CONCLUSION
The research findings of this study conclude that Curvularia sp may be used as one of the fungal isolates for the production of lovastatin in mushroom extract medium. This novel method of using these fungi for lovastatin was reported the first time and further studies may be done to enhance productivity.
ACKNOWLEDGEMENT
We wish to express sincere gratitude to Fungal Biodiversity and BioResources Research Laboratory at Bannari Amman Institute of Technology for providing facilities for the successful completion of research work.
Authors contribution
MS - Batch fermentation, recovery of the product, report writing and edition
KPK - Biostatistics for Species Diversity and report writing
KR, HR and GG –Screening, report writing.
Conflict of Interest: Nil
Source of Funding: Nil
References:
-
Abd Rahim MH, Hasan H, Montoya A, Abbas A. Lovastatin and (+)-geodin Production by Aspergillus terreus from crude Glycerol. Eng Life Sci. 2015;15(2):220-228.
-
Akpotu MO, Eze PM, Abba CC, Nwachukwu CU, Okoye FBS, Esimone CO. Metabolites of Endophytic Fungi Isolated from Euphorbia hirta Growing in Southern Nigeria. Chem Sci Rev Lett 2017;6(21):12-19.
-
Atli B, Yamac M, Yildiz Z. Optimization of Submerged Fermentation Conditions for Lovastatin Production by the Culinary-Medicinal Oyster Mushroom, Pleurotusostreatus (Higher Basidiomycetes). Int J Med Mush 2013;15(5):487-495.
-
Belwal C, Goyal PK, Balte A, Kolhe S, Chauhan K, Rawat AS, Vardhan A . Isolation, Identification, and Characterization of an Unknown Impurity in Lovastatin EP. Sci Pharma 2013;82(1):43-52.
-
Bobek P, Ozdín L, Galbavý S. Dose- and time-dependent hypocholesterolemic effect of oyster mushroom (Pleurotus ostreatus) in rats. Nutrition 1998;14 (3):282–286.
-
Lopez C, Sanchez Perez JA, Fernández Sevilla JM, Acien Fernandez FG, Molina Grima E & Chisti Y.Production of Lovastatin by Aspergillus terreus: effects of the C: N ratio and the Principal Nutrients on Growth and Metabolite Production. Enzy Microb Tech 2003;33(2-3):270-277.
-
Chang YN, Huang JC, Lee CC, Shih IL, Tzeng YM. Use of response surface methodology to optimized medium for the production of lovastatin by Monascusruber. J Enzy Microbio 2002;30(7): 889-894.
-
Chi SM, Wang Y, Zhaw Y, Pu JX, Du, X, Liu JP, et al. A New cyclopentanone derivative from Euphorbia hirta. Chem Nat Compd 2012;48(4):577-579.
-
Dobranic JK, Johnson IA, Alikhan QR. Isolation of Endophytic Fungi from Eastern Larch (Larix Laricina) Leaves from New Brunswick, Canada. Can J Microbiol 1995; 41(2):194-198.
-
Eyob CC, Raju KC. Endophytic Mycoflora and Their Bioactive Compounds from
Azadirachta indica: A Comprehensive Review. J Mycol 2018;4(2):1-12.
-
Fischer J, Robin G. Analogue-based Drug Discovery. John Wiley & Sons. 2006;4:472.
-
Fisher PJ, Petrini O. A comparative study of fungal endophytes in xylem and
bark of Alnus Species in England and Switzerland. Mycol Res 1990;94(3):313-349.
-
Gangadevi V, Muthumary J. Taxol an anticancer drug produced by an endophytic fungus Bartaliniarobillardoides Tassi, isolated from a medicinal plant, Aegle marmelos Correa ex Roxb. World J Microbio Biotech 2008;24(5):717-724.
-
Hajjaj H, Niederberger P, Duboc P. Lovastatin Biosynthesis by Aspergillus terreus in a Chemically Defined Medium. Appl Envt Microbio 2001;67(6):2596-2602.
-
Hirama M, Iwashita M. Total synthesis of (+)-monacolin K (mevinolin). Tetrahedron Lett 1983; 24(17):1811-1812.
-
Karthika C, Sharmila G, Muthukumaran C, Krishnan M. Utilization of Whey Powder as an Alternate Carbon Source for Production of Hypocholesterolemic Drug by Aspergillus terreus MTCC 1281. J Food Sci Biotechnol 2013;22(5):1-7.
-
Kumar DSS, Hyde KD. Biodiversity and Tissue-Recurrence of Endophytic Fungi in Tripterygium wilfordii. Fungal Divers 2004;17(1):69-90.
-
Kumar DSS, Lau CS, Wan JMF, Yang D, Hyde KD. Immunomodulatory compounds from Pestalotiopsis leucothes (HKUCC 10197), an Endophytic Fungus of Tripterygium wilfordii. Life Sci 2005;78(1):147-156.
-
Kumar MS, Jana SK, Senthil V, Shashanka V, Kumar SV, Sadhukhan AK. Repeated Fed-Batch Process for Improving Lovastatin Production. Proc Biochem 2000; 36(1):363-368.
-
Kumar MS, Kumar PM, Sarnaik HM, Sadhukhan AK. A rapid technique for screening of lovastatin-producing strains of Aspergillus terreus by agar plug and Neurospora crassa bioassay. J Microbiol Meth 2000;40(1):99-104.
-
Lai LS, Hung CS, Lo CC. Effects of lactose and glucose on the production of itaconic acid and lovastatin by Aspergillus terreus ATCC 20542. J Biosci Bioeng 2007;104(1):9-13.
-
Lai, LST, Pan CC, Tzeng BK. The influence of medium design on lovastatin production and pellet formation with a high producing mutant of Aspergillus terreus in submerged cultures.Proc Biochem 2003;38(9):1317-1326.
-
Lee CL, Wang JJ, Pan TM.Synchronous Analysis Method for Detection of Citrinin and the Lactone and Acid forms of Monacolin K in Red Mold Rice. J Biochem 2006;89(89):669-677.
-
Luthra U, Singh N, Tripathi A, Vora S, Bhosle V. Media Optimization for Lovastatin Production by Statistical Approach using Aspergillus terreus by Submerged Fermentation. Int J Res Med Sci 2015;3(2): 4520-4528.
-
Parija SC, Shivaprakash MR, Jayakeerthi SR.Evaluation of Lacto-phenol cotton blue (LPCB) for detection of Cryptosporidium, Cyclospora and Isospora in the wet mount preparation of stool. Acta Trop 2003;85(3):349-354.
-
Senthamarai M, Kannan KP.Optimization of lovastatin production by Fusarium nectrioides (MH173849) using response surface methodology and fuzzy logic system. J Environ Biol 2019;40(5):1036-1044.
-
Samiee SM, Moazami N, Haghighi S, Mohseni FA, Mirdamadi S, Bakhtiari MR. Screening of Lovastatin Production by Filamentous Fungi. Iran Biomed J 2003;7(1):29-33.
-
Sharma S, Kumar S. Phyllanthus reticulatus Poir. – An important medicinal plant: A review of its Phytochemistry, Traditional uses and Pharmacological properties. Int J Pharm Sci Res 2013:4(7);2528-2534.
-
Su YC, Wang JJ, Lin TT, Pan TM. Production of the secondary metabolites γ-aminobutyric acid and monacolin K by Monascus. J. Ind Microbiol Biotech 2003;30(1):41-46.
-
Suryanarayanan TS, Kumaresa V and Johnson JA. Foliar fungal endophytes from two species of the mangrove Rhizophora. Can J Microbiol 1998;44(10):1003-1006.
-
Vagelos PR, Galambol L. Medicine, Science and Merck. Cambridge University Press, Cambridge, United Kingdom.2004;211-301.
-
Wang F W., Jiao R H., Cheng A B, Tan S H, Song Y C. Antimicrobial potentials of endophytic fungi residing in Quercus variabilis and brefeldin A obtained from Cladosporium sp. World J Microbiol Biotech 2007;23:79–83.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License