IJCRR - 13(3), February, 2021
Pages: 94-98
Date of Publication: 03-Feb-2021
Print Article
Download XML Download PDF
Clinical Connotation of HPV and Effect of Various Treatment Modalities in Disease Free Survival for the Oropharyngeal Cancer
Author: Sachin Mohite, Vimal Vibhakar, Meenakshi Kekre, Manish Bhargava
Category: Healthcare
Abstract:Background: Oropharyngeal cancer incidence has been increasing all over the world in the last decades despite advances in various surgical and non-surgical therapies for carcinoma treatment. The incidence of other head and neck cancers are decreasing in the world. It is now established that HPV can be contributed as a part of that increase. Objective: The present trial was aimed to investigate if the oropharyngeal cancer incidence increases with HPV, also to evaluate if the proportion of HPV-positive oropharyngeal cancer patients continues to increase, and if different treatment therapies given to patients with HPV-positive oropharyngeal cancer affect prognosis. Methods: The study was carried out 468 cases of oropharyngeal cancer,88 biopsy samples pre-treatment were available and were analysed with the PCRfrom year 2019-2020 hospital records of various cancer tertiary care centres of the Indian continent. Cases positive for PCR and p16 by immunohistochemistry were finally included in the study. Also, the detection of E7 RNA and E6 of HPV-16 was done, as these are the oncogenes that seem necessary for the oncogenesis process and also contribute to the establishment of the fact that HPV can lead to the carcinomas of the base of the tongue and oropharyngeal base. P16 immunohistochemistry was added as the diagnostic criteria to avoid misdiagnosis/overdiagnosis. Results:66 samples were tested positive for HPV 16, one sample for HPV 33, one sample for HPV 59, and one sample for HPV 35. Three samples out of these 88 were not able to be detected for HPV. mRNA expression for E6 and E7 HPV-16 was detected in all samples except 1 sample. The 88 positive patients were treated with radiotherapy (40), chemoradiotherapy (20), or accelerated radiotherapy (28). No difference was noticed concerning the overall survival rates with the three different treatment modalities used. Conclusion: The results showed that the percentage of HPV positive patients with oropharyngeal cancer increased exponentially from one year to another in consecutive years. No difference was noticed about the overall survival rates with the three different treatment modalities used including radiotherapy, chemoradiotherapy, or accelerated radiotherapy.
Keywords: Oropharyngeal Cancer, Human Papilloma Virus (HPV), Squamous Cell Carcinoma, Radiotherapy, Chemoradiotherapy, Accelerated Radiotherapy
Full Text:
INTRODUCTION
A majority of oropharyngeal cancers are squamous cell carcinoma (SCC).1 In south Asian countries, oropharyngeal cancer is the most common cancer.2 The other sites include the base of the tongue, soft palate, posterior pharynx wall, and uvula. Tonsils and base of tongue are both parts of Waldeyers ring and have similarities in histological and morphological aspects with the lymphoid tissue.3 Waldeyer’s ring containsSubepithelial and submucosal lymphatic tissues localized in the pharynx. Waldeyer’s ring comprises of the tubal, pharyngeal, palatine, and lingual tonsils. Pharyngeal, palatine and lingual tonsils are part of the secondary immune system. They are exposed to the antigens through epithelial layers. These epitheliums are composed of squamous cells. Various microorganisms infect the tonsils including adenoviruses, Epstein-Barr virus, herpes simplex virus, parainfluenza virus, respiratory syncytial virus, and influenza A and B viruses.4,5In the past decade, human papillomaviruses (HPVs) has shown to infect the oropharyngeal epithelium. As seen in the other mucosal sites, HPV has shown malignant transformation in the oropharyngeal epithelial region.6,7
Oropharyngeal Cancers are topographically classified according to their site of origin as the floor of the mouth, lip, tongue, retromolar triangle, buccal mucosa, the base of the tongue, hard palate, soft palate, oropharyngeal areas, and pharyngeal folds.8 At diagnosis, oropharyngeal carcinomas are classified on the TNM basis (2002) as the size of the primary tumour (T), presence, size, number, and localization of regional metastasis (N) and presence of distant metastasis (M). Treatment of cancers related to oropharyngeal regions is primarily done using either one or a combination of the mentioned techniques. These techniques include radiotherapy with/without chemotherapy, External radiotherapy, interstitial radiotherapy (brachytherapy), Chemotherapy, Drug treatment (Cisplatin, 5, fluorouracil, and sometimes docetaxel), concomitant chemotherapy (administered during radiotherapy using drugs like cetuximab weekly). Adjuvant chemotherapy is sometimes combined with radiotherapy and may consist of cetuximab or cisplatin. Historically, surgical treatment was more common 20-30 years ago and now administered when other therapies fail to respond.9
Human Papilloma Virus (HPV) was first identified in 1949, using electron microscopy. In the 1970s, it was found that HPV is a virus family capable of causing different diseases. To date, more than 120 HPV types have beenidentified10 and around 15 HPV types are known as “high risk”, due to to their role in cancer development including oropharyngeal carcinoma. HPV infection, in tonsils, is restricted to the basal cells in the epithelial layers of the mucosa. This malignant transformation in the epithelial cells is attributed to the mutations in the p53 gene and on chromosome 9, p21-22 is lost early in carcinogenesis, this leads to the inactivity of the tumour-suppressing gene p16.11Head and neck tumours with the presence of HPV show less expression destructive type of p53 due to its inactivation by the E6 oncoprotein. Earlier palatine tonsils cancer was the most common oropharyngeal carcinoma. However, the incidence of oropharyngeal cancer has significantly decreased in many countries due to the new international classification of disease (ICD) guidelines since 1993.10,11
In the early stages, the disease presents no symptoms. Clinical symptoms and signs of oropharyngeal cancer include difficulties in swallowing, pain (especially in the ear), palpable firmness, visible lesions in the tonsil, oropharyngeal asymmetry, neck mass, and unexplained weight loss.12 Patients with oropharyngeal cancer often present with unilateral sore throat or earache but can also present with a neck mass depicting nodal metastasis.10,11 The risk factors for the oropharyngeal carcinomas are smoking and alcohol also is seen in the oropharyngeal cancer patient. With the increase in oropharyngeal SCC, the frequency of smokers has declined, therefore it can be concluded that additional risk factors probably exist for oropharyngeal malignancy.13,14 Studies during the recent past suggest that oropharyngeal SCCs may be associated with carcinogenic HPV infections.15
It is shown in the literature recently that patients with HPV associated anogenital cancer had a higher risk (4.3 fold) of oropharyngeal SCC.13Also, husbands of HPV associated cervical cancer patients showed an increased risk for oropharyngeal cancer. This suggests that HPV can be involved in oropharyngeal cancer. Further, HPV associated oropharyngeal carcinomas are found in transplant recipients.16,17 The mode of HPV entry to the oropharyngeal tissue is not yet known. Oropharyngeal crypt epithelium is known to capture and process antigens, which might be an entry portal of the virus to the basal cells. Also, oropharyngeal tissue could act as a reservoir of HPV in the digestive tract. This can be attributed to the fact that oral samples collected by oral rinse show much higher HPV levels compared with the swabs. HPV persistence in oropharyngeal tissue is also seen.
The present trial was aimed to investigate if the oropharyngeal cancer incidence increases with HPV, also to evaluate if the proportion of HPV-positive oropharyngeal cancer patients continues to increase, and if different treatment therapies given to patients with HPV-positive oropharyngeal cancer affect prognosis.
MATERIALS AND METHODS
The study was carried out 468 cases of oropharyngeal cancer, 88 biopsy samples pre-treatment were available and were analysed with PCR from the year 2019-2020 hospital records of various cancer tertiary care centres of the Indian continent. Following data for the patients included in the study was recorded:
1) Demographic Data of the patient: a number for identification of the subject, age, sex, address.
2) Malignancy status data: tumour site, Diagnosis basis, diagnosis date, histological type, reporting pathology, tissue specimen.
3) Follow-up record: death, cause of death.
To assess the prevalence of the HPV, all the patients diagnosed with the oropharyngeal squamous cell carcinoma between the mentioned periods were identified using the hospital data. For all the included patients, pretreatment biopsy samples were collected to be analyzed with PCR, and tumour characteristics were also recorded, 88 samples were available. Out of the included 468 patients, subjects who were confirmed with the cancer diagnosis via PCR and positive for p16 by immunohistochemistry were finally included in the study. Demographic data, clinical data, and the follow-up data were then noted from the medical records.
For the detection of HPV DNA, PCR analysis was done. Also, the detection of E7 RNA and E6 of HPV-16 was done, as these are the oncogenes that seem necessary for the oncogenesis process and also contribute to the establishment of the fact that HPV can lead to the carcinomas of the base of the tongue and oropharyngeal base. P16 immunohistochemistry was added as the diagnostic criteria to avoid misdiagnosis/overdiagnosis.
For the detection of DNA, tumour sections were embedded in paraffin after fixing in formalin. The presence of HPV and its type in samples was detected using PCR using the manufacturer's directions. All PCR samples were stained with ethidium bromide dye to aid in visualization.
E6 and E7 mRNA was also analyzed to detect if HPV found was active transcriptionally. RNA extraction was done from the oropharyngeal tumour sections embedded in the paraffin using the kit for RNA isolation (as per the instructions by the kit manufacturer). Samples were tested and marked as either negative or positive for E6 and E7 mRNA. However, to detect if different treatment therapies are given to patients with HPV-positive oropharyngeal cancer affect prognosis, p16 analysis was done instead ofE6 and E7 mRNA. P16 analysis was done to determine biologically active HPV. The detection of p16 was done using the immunochemistry assay using primary monoclonal mouse anti-human p16INK4a antibody. Immunohistochemical staining was done and evaluated.
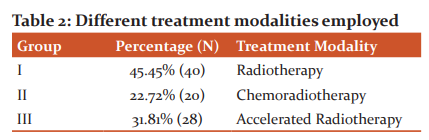
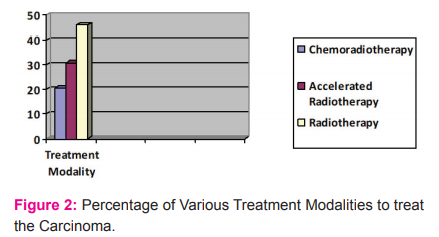
The 88 positive patients were treated with either radiotherapy (40), chemoradiotherapy (20), or accelerated radiotherapy (28). The patients treated with the three different treatment modalities were in different stages of the oropharyngeal carcinoma. The patients at a higher stage were treated with chemoradiotherapy as compared to the other two treatment modalities.
RESULTS
Out of these 468 patients, 88 samples were available pre-treatment for using the PCR for HPV detection using biopsy. 66 samples were tested positive for HPV 16, one sample for HPV 33, one sample for HPV 59, and one sample for HPV 35. Three samples out of these 88 were not able to be detected for HPV. mRNA expression for E6 and E7 HPV-16 was detected in all samples except 1 sample.
The mean age of the patients detected positive for tumour were relatively younger with the mean age of 56 years whereas negative patients were within the age group of 64 years. The tumours tested positive for HPV were found poorly differentiated on examination.
On comparing the data year-wise, the percentage of the patients found positive for HPV oropharyngeal cancer is depicted in Table 1& Figure 1.
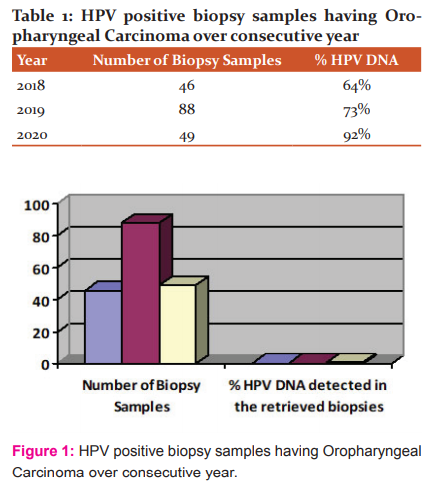
The results showed that the percentage of positive patients increased exponentially from one year to another from 64% to 92% of the sample number increased from one year to next year. The percentage however increased in the next year despite a decrease in the sample numbers obtained from 88 to 49. The 88 positive patients were treated with either radiotherapy (40), chemoradiotherapy (20), or accelerated radiotherapy (28). The patients treated with the three different treatment modalities were in different stages of the oropharyngeal carcinoma. The patients at a higher stage were treated with chemoradiotherapy as compared to the other two treatment modalities.
Concerning the treatment modalities used in the patient with the oropharyngeal carcinoma, 88 patients were treated who had pre-treatment biopsy samples available for analyzing HPV. The 88 positive patients were treated with radiotherapy (40), chemoradiotherapy (20), or accelerated radiotherapy (28. The patients treated with the three different treatment modalities were in different stages of the oropharyngeal carcinoma. The patients at a higher stage were treated with chemoradiotherapy as compared to the other two treatment modalities (Table 2 & Figure 2).
No difference was noticed concerning the overall survival rates with the three different treatment modalities used. On comparing chemotherapy against the radiotherapy group, again no significant difference was seen in either the death rate or survival rate. The study also analyzed if the subject group treated with the chemoradiotherapy had lesser distant metastases when compared to the other two treatment modalities. The result showed no significant difference in the distant metastases between the groups.
DISCUSSION
The present trial was aimed to investigate if the oropharyngeal cancer incidence increases with HPV, also to evaluate if the proportion of HPV-positive oropharyngeal cancer patients continues to increase, and if different treatment therapies given to patients with HPV-positive oropharyngeal cancer affect prognosis. The proportion of patients with oropharyngeal cancer with the positive presence of the HPV in the samples is increasing day by day.18 For the present study, there were differences in the age of the patients in cases with positive oropharyngeal cancer.19,20 These findings were based on the studies by Fakhry C et al and Hammarstedt L. et al. in 2006.
While evaluating the Treatment outcomes with the three treatment modalities, i.e, conventionally fractionated radiotherapy, accelerated radiotherapy or chemoradiotherapy, no significant difference in the survival rates was seen with any of the three therapies. These findings can be contributed to the small sample size of the study population (n=88), and these findings need to be further evaluated. In a similar study conducted by Ang et al21 in 2010, authors found no significant difference in the survival rates for the HPV positive patients with oropharyngeal cancer, comparing standard fractionated radiotherapy with accelerated radiotherapy. However, in the study by Ang et al high dose of cisplatin was given to all the patients.
The present study used HPV E6, E7 mRNA, and p16 immunohistochemistry for the analysis of HPV in the cases with oropharyngeal cancer. These methods have been. The use of oncogene HPV E6 is shown to be the gold standard when frozen biopsies are used by Smeets S.J et al22 in 2007, this supports the use of this oncogene assessment in the present study. Shi W et al23 in their 2007 study favoured the use of GP5+/6+ PCR, HPV16 E6/E7 mRNA, HPV16 in situ hybridization (ISH), and p16 immunohistochemistry (IHC) for HPV detection, favouring the present study.
Various other studies by different authors including the studies by Weinberger et al.24 in the year 2006 favoured using the combination of the techniques used in the present study to detect HPV in the subjects with oropharyngeal cancer. The most feasible method should be selected considering the factors like specificity, sensitivity, cost, and reproducibility for the selected technique. Although the present study detected HPV DNA using PCR, another study by Weinberger P.M. et al in 2004 has shown that mere detection of HPV DNA has little or no prognostic value as PCR does not differentiate transcriptionally active and inactive cases.25 Hence, the present study utilized E6 and E7 mRNA in the biopsies which suggest that the detected virus is a transcriptionally active and relevant parameter to judge carcinogenesis.
The present study was a retrospective study and hence limits the reliability and personal habits of the included subjects such as smoking, weight, malnutrition, and ecological environment. This warrants the conduction of more prospective studies with a longer monitoring period and sample size. This will also help in overcoming the selection bias and difference in the clinical appearance. The use of the HPV vaccine and its effect shall also be considered. Antiviral therapies targeted against HPV and not towards oropharyngeal cancer shall also be tested for their effect on HPV associated oropharyngeal cancers.
CONCLUSION
The results showed that the percentage of HPV positive patients with oropharyngeal cancer increased exponentially from one year to another in consecutive years. No difference was noticed concerning the overall survival rates with the three different treatment modalities used including radiotherapy, chemoradiotherapy, or accelerated radiotherapy. Conduction of more prospective studies with longer monitoring period and the larger sample size is required. This will also help in overcoming the selection bias and difference in the clinical appearance. The use of the HPV vaccine and its effect shall also be considered. Antiviral therapies targeted against HPV and not towards oropharyngeal cancer shall also be tested for their effect on HPV associated oropharyngeal cancers. More research is required to better define the most accurate and feasible diagnostic method for HPV diagnostics in the clinical setting.
References:
-
The National Board of Health and Welfare, Cancer incidence in Sweden 2007, Swedish Cancer Registry. 2008.
-
Licitra L. Cancer of the oropharynx. Crit Rev Oncol Hematol 2002;41:107-122.
-
Boyle LB. World Cancer Report 2008. International Agency for Research on Cancer (IARC), 2008.
-
Mellin H, Dahlgren L, Munck-Wikland E. Human papillomavirus type 16 is episomal and a high viral load may be correlated to better prognosis in tonsillar cancer. Int J Cancer 2002;102:152–158.
-
Mellin H. Human papillomavirus in tonsillar carcinoma. Stockholm, Sweden: Karolinska University Press, 2002.
-
Mork J, Lie AK, Glattre E. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med 2001;344:1125–1131.
-
Paz IB, Cook N, Odom-Maryon T, et al. Human papillomavirus (HPV) in head and neck cancer. An association of HPV 16 with squamous cell carcinoma of Waldeyer’s ring. Cancer 1997;79:595–604.
-
Barnes L, Johnson JT. Pathologic and clinical considerations in the evaluation of major head and neck specimens resected for cancer. Part I. J Pathol Annu 1986;21:173–250.
-
Rampino M.Efficacy and feasibility of induction chemotherapy and radiotherapy plus cetuximab in head and neck cancer. Anticancer Res 2012;32:195-9.
-
Bernard HU, Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 2010;401(1):70-79.
-
Kim, SH.., HPV integration begins in the tonsillar crypt and leads to the alteration of p16, EGFR and c-myc during tumour formation. Int J Cancer, 2007;120:1418-1425.
-
Beaty MM, Funk GF, Karnell LH. Risk factors for malignancy in adult tonsils. Head Neck 1998;20:399–403.
-
Frisch M, Biggar RJ. The aetiological parallel between tonsillar and anogenital squamous cell carcinomas. Lancet 1999;354:1442–1443.
-
Hemminki K, Dong C, Frish M. Oropharyngeal and other upper aerodigestive tract cancers among cervical cancer patients and their husbands. Eur J Cancer Prev 2000;9:433–437.
-
Syrjänen K, Syrjänen S. Papillomavirus infections in human pathology. London: Wiley, 2000.
-
Syrjänen KJ. HPV infections and oesophageal cancer. J Clin Pathol 2002;55:721–728.
-
Manual of the international statistical classification of diseases, injuries, and causes of death. Geneva: World Health Organisation. 1979.
-
Chaturvedi A. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011;29:4294-4301.
-
Hammarstedt L. Human papillomavirus as a risk factor for the increase in the incidence of oropharyngeal cancer. Int J Cancer 2006;119:2620-2623.
-
Fakhry C. Gillison ML. Clinical implications of human papillomavirus in head and neck cancers. J Clin Oncol 2006;24:2606-2611.
-
Ang, KK. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24-35.
-
Smeets SJ. A novel algorithm for reliable detection of human papillomavirus in paraffin-embedded head and neck cancer specimen. Int J Cancer 2007;121:2465-2472.
-
Shi W. Comparative prognostic value of HPV16 E6 mRNA compared with in situ hybridization for human oropharyngeal squamous carcinoma. J Clin Oncol 2009;27:6213-6221.
-
Weinberger PM. Molecular classification identifies a subset of human papillomavirus-associated oropharyngeal cancers with favourable prognosis. J Clin Oncol 2006;24:736-747.
-
Weinberger PM, Prognostic significance of p16 protein levels in oropharyngeal squamous cell cancer. Clin Cancer Res 2004;10:5684-5691.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License