IJCRR - 8(4), February, 2016
Pages: 61-67
Date of Publication: 21-Feb-2016
Print Article
Download XML Download PDF
THE EFFECT OF MUCUNA PRURIENS SEED EXTRACT ON PANCREAS AND LIVER OF DIABETIC WISTAR RATS
Author: Rajesh R., S. Arunchandra Singh, K. Anandraj Vaithy, K. Manimekalai, Dhananjay Kotasthane, S. S. Rajasekar
Category: Healthcare
Abstract:Aims and Objectives: The study was to evaluate the remedial effect of alcohol extract of Mucuna pruriens seeds on pancreas and liver of Streptozotocin induced diabetic rats. Materials and Method: Five day old neonate wistar rats (n -24) were used for the study. Out of this, 6 rats were kept as Normal control i.e. Group A. Remaining 18 rats were made diabetic by intraperitoneal injection of streptozotocin (65mg/kg body weight). After 12 weeks, they were divided equally into 3 groups i.e. Group B - Diabetic control, Group C- Mucuna Pruriens group (200mg/kg), Group D - Glibenclamide group (1 mg/kg body weight). Drugs were administered orally for 28 days in group C and D. Blood Glucose Level was monitored once in a week during this period. On completion of drug treatment period, animals were sacrificed for the collection of blood and visceral organs to carry out histological and biochemical investigations. Results: The Mucuna Pruriens seed extract (200mg/kg) were effectively controlled blood glucose levels in diabetic rats. Serum insulin and cholesterol levels were significantly improved when compared to diabetic group (p >0.05). In pancreas, the islets showed increase in beta cell mass and reduced necrotic changes. Liver functions were partially restored and hepatocytes showed minimal necrotic changes. Conclusion: Mucuna pruriens seeds are capable of exerting positive structural changes in pancreas & liver through its antioxidant and antidiabetic properties.
Keywords: Glibenclamide, Islets of langerhans, Intraperitoneal, H&E staining
Full Text:
INTRODUCTION
Diabetes mellitus is considered as one of the most serious threat to human in the twenty first century(1). it is a systemic disease characterized by consistent hyperglycemia by damage to the liver and pancreas .The word diabetes is derived from the Greek word ‘diabetes’ meaning 'siphon' - 'to pass through' and the Latin word ‘mellitus' meaning honeyed or sweet(2). This is because the presence of excess sugar in blood and urine. Sushrutha (6th century BC) identified diabetes and named it as ‘Madhumeha' meaning honey in the urine(3). Of the two types of diabetes mellitus, Type II is more prevalent than the Type I variant. The underlying pathology is defect in the mechanism of insulin secretion and insulin resistance(4) by the damage in the beta cells of pancreatic islets and disturbances in the liver, which hampers carbohydrate, protein and lipid metabolism(5). The free radical formation is also playing a pivotal role in the progress of diabetes (4). The common drugs used in the management of Type 2 Diabetes in the modern medicine are Metformins, Biguinides, Sulphonylurea etc. However, the efficacy of these drugs is limited in long term due to its side effects. In Ayurvedic med icine, Momordica Charantia, (Bitter gourd)(6)(7)(8) Helicterus Isora(9), Curcurmin(10), Trigonella foenum graecum (Fenugreek)(11)are used for the treatment for diabetes. Researches on these herbal drugs documented minimal adverse effects and prevention of the secondary complications (6). Mucuna Pruriens (Linn), a leguminous plant which belongs to fabaeceae family, is found in the tropical regions of India, Africa and West Indies(12). The seeds and leaves of this plant are well known and widely used for the treatment of Erectile dysfunctions(13)(14)(15), Epilepsy and Parkinsonism in Ayurveda. The seeds are rich in fiber content and antioxidants (27). Previous researchers like Anusha et al, Enechi et al and Majekudunni et al studied the biochemical aspects of Mucuna seeds in Type I diabetes. None of these works examined the histological perspective to reveal the structural modifications in pancreas and liver, Moreover no studies have been reported on Type II diabetes in this drug. The present study was undertaken with a novel aim to explore the histological and biochemical aspects of medicinal properties of Mucuna Pruriens seeds in Type II diabetic rats.
MATERIALS AND METHODS
Source of plant extract The ethanolic extract of Mucuna pruriens was procured from Herbal Research Department, VPSV Ayurveda College. Kottakkal, Kerala. Experimental Study Design The experimental protocol was approved by Institutional Animal Ethics Committee (IAEC) of Mahatma Gandhi Medical College and Research Institute, SBV University, Puducherry (686/02/a/CPCSEA). Five-day-old neonate wistar rats (n-24) were received from the Central Animal House of Mahatma Gandhi Medical College and Research Institute. The neonate rats were made diabetic by a single dose i.p. injection of streptozotocin (Sigma Aldrich, U.S.A) (65 mg/kg body weight) dissolved in freshly prepared citrate buffer (0.1M, pH-4.5) (18) (19). After 6 weeks, blood sugar levels [BGL] were measured by using a one-touch glucometer (AccuChek, Roche Diagnostics, USA) and animals showing value > 150mg/dl of BGL were selected for the study. After 12 weeks, rats were classified into four groups of six animals each. Food and water were provided ad libitum to the rats. The groups are as follows Group A – Normal control (Non diabetic rats); Group B – Diabetic control; Group C – Experimental drug (Mucuna); Group D-Standard drug (Glibenclamide).
Mode of feeding of drugs The Mucuna Pruriens (200mg/kg body weight) and glibenclamide (1 mg/kg body weight) administered orally for 28 days in Group C and D respectively. Group A and B were given equal volume of purified water.
Assessments of Biochemical Parameters: The Total Body Weight and Fasting Blood Glucose Levels [FBGL] were measured once in a week during the drug treatment period by using standard glucometer strips with working principle of lactate dehydrogenase method. After the completion of experiment period, all the rats were deprived of food overnight and then sacrificed by painless cervical dislocation under mild chloroform anesthesia. The blood samples were collected in a heparinized tube for biochemical assessments. The parameters evaluated were (A) Serum insulin level-using a standard commercial diagnostic kit with reference values. (B) High Density Lipoprotein , Low Density Lipoprotein and Total Protein were assessed by Spectrophotometric method as per standard laboratory guidelines(20).
Histological studies
Pancreas and Liver were carefully dissected out and fixed in 10% neutral buffered formalin for 48 hours. After processing, the tissues were embedded in paraffin wax. Histological sections were cut at 5micron thickness by using a semiautomatic rotary microtome and stained with H and E. Photographic and Light microscopic studies are carried out by using Olympus research microscope (CX41RF), Germany.
Statistical analysis One-way analysis of variance (ANOVA) (SPSS 15.0) and the related post- hoc test were applied for data investigation. All the observed data are entered, tabulated and shown as Mean ± SD range and P value < 0.05 were identified as significantly different.
RESULTS
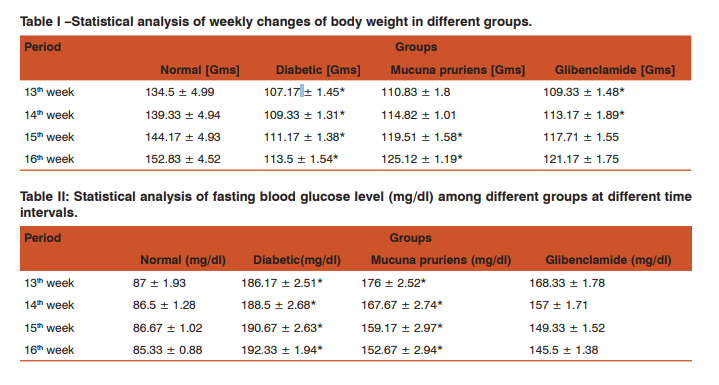
A. Blood Glucose Level and Bodyweight. During the experimental period, the animals among the experimental groups and diabetic control groups were ill looking and showed marked weight loss. In diabetic group, there was a significant increase in the blood glucose level on comparison with the normal rats (P-value <0.05). Mucuna pruriens seed extract treatment resulted in statistically significant reduction of blood glucose levels when compared to diabetic control. Simultaneously treatment with glibenclamide showed more significant hypoglycemic activity at the initial phase of two weeks and in the latter two weeks, Mucuna pruriens extract showed amplified hypoglycemic activity.
The total body weight was gradually increased among the Mucuna pruriens and glibenclamide groups. Mucuna pruriens were found to be more effective in gaining body weight in diabetic rats (Table.1).
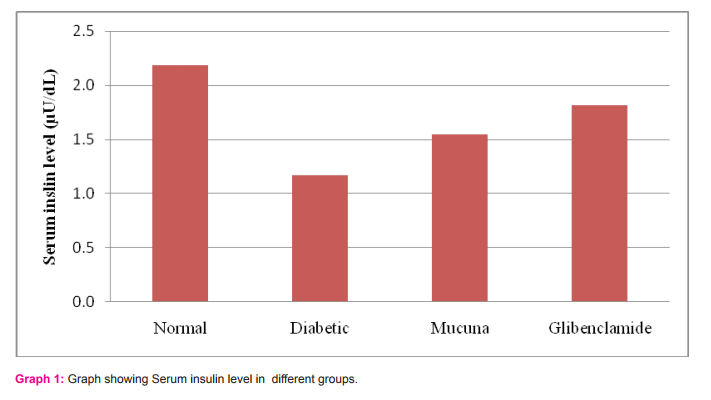
B. Biochemical parameters I. Serum insulin Serum insulin level was significantly enhanced in both the experimental groups (P value < 0.05). The potency of glibenclamide to improve the insulin level was observed to be more significant in comparison to Mucuna Pruriens (Table -II). II. Lipid profile The lipid profile analysis of diabetic group showed decrease in High-Density Lipoprotein (HDL) and Total Protein levels (TP) whereas a clear increase in Low-Density Lipoprotein (LDL). These changes are moderately reversed in Mucuna pruriens and glibenclamide groups (Table-III).
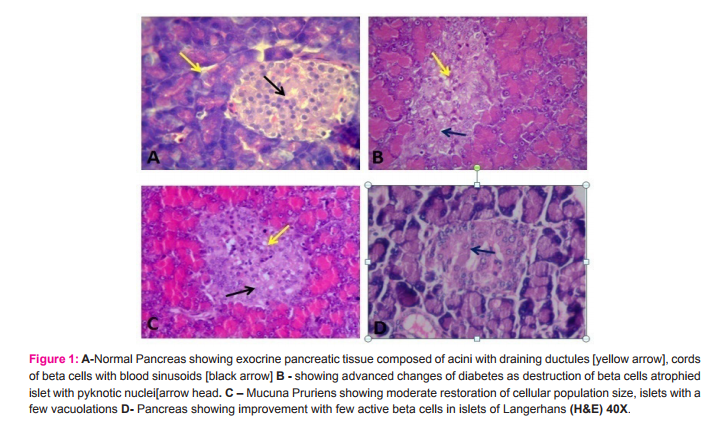
C. Histopathological changes I. Pancreas Group A: Histology of islet cell clusters of normal control group, sacrificed on 28th day, showed normal histology of exocrine and endocrine pancreas. The islet of Langerhans appeared as pale-stained rounded clusters with normal cytoarchitecture. On high power view, the islets appeared in loose clusters comprising of polygonal cells arranged in cords and interspersed with numerous blood capillaries. Group B: In diabetic group, a complete distortion of architecture and reticular changes were observed. Other findings observed were: 1) Infiltration of inflammatory cells (mostly lymphocytes) in the islets and surrounding connective tissues. 2)
Severe necrotic changes in the central part of the islets involving apparent destruction of the beta cells, dilated nucleus with mild fragmentation and congestion of the blood vessels. Exocrine part appeared highly eosinophilic. Group C: Histological analysis of endocrine part showed improvement in size and shape of islets with restoration of beta cells, better vasculature, decreased inflammatory cell infiltrations and the exocrine part showed enhanced cellular architecture. Group D: Islets showed evidence of reduced number of inflammatory cellular infiltrations, symmetrical vacoulations, moderate level of cellular necrosis and a few active beta cells in the central area of islets clusters with better cellular architecture.
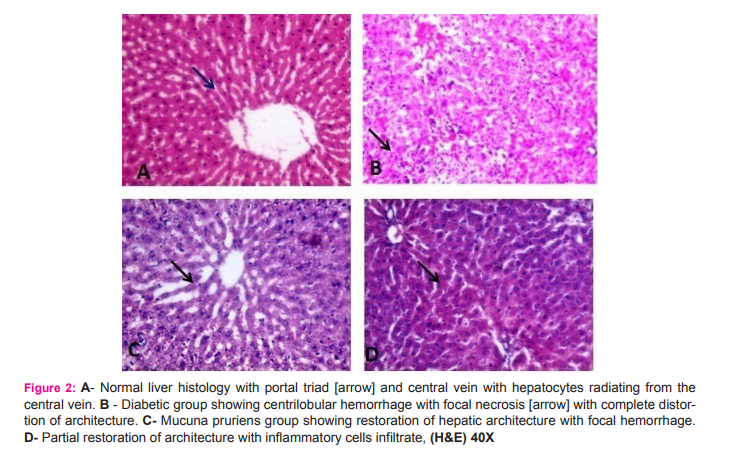
Group B: The hepatic sinusoids were non-radiating and tend to be wider and interrupted in the untreated STZ diabetic rats. Hepatocytes were degenerated with loss of architecture with decreased number of central nuclei and mild steatosis with geographical necrosis and inflammatory cell infiltrates. Group C: Treatment with extracts of Mucuna pruriens caused partial reversal in the damage observed with diabetic group. The nuclei of the hepatocytes were rather faint with reduced inflammatory cell infiltrates with minimal fatty changes. Group D: Treatment with glibenclamide showed almost similar observations in Group C. The hepatocytes were distinctly outlined together with their nuclei, reduced necrotic changes and vacoulations, implying an increase in activity of the cells.
DISCUSSION
Diabetes mellitus is a chronic metabolic disorder which affects almost all systems of the body and the management includes various treatment modalities (5). Apart from the currently available drug regimens for management of diabetes, a wide range of drugs extracted from plant species were examined for their possession of anti diabetic properties(5), (6) .These drugs found to be relatively less toxic with minimal adverse effects in comparison with common allopathic drugs. On these backgrounds, the present study was designed to evaluate the hypoglycemic effects of Mucuna pruriens seed extract on the structural changes in liver and pancreas of neonatal Streptozotocin induced diabetic rats.
Streptozotocin (STZ) carrying a chemical formula 2-Deoxy -(3- methyl -3-nitrosurea)-1-D-glucopyranose, is a naturally occurring compound synthesized by the soil bacterium Streptomyces Achromogenes, which selectively destroys beta cells by means of a glucose transporter (GLUT2) and generates alkylation of DNA resulting in rapid necrosis. Hyperglycemia and hyperlipidemia are considered as most critical problems in Diabetes mellitus. The observations of the present study showed the effectiveness of STZ in producing persistent hyperglycemia in experimental animals and are in concordance with studies performed by Abdollahi et al., in 2008 Lee et al., in 2009.
Earlier pharmacological studies shows that the ethanolic extract of the Mucuna seeds are rich source of dietary fibres and antioxidants and it contains anti diabetic components like saponins, squalenes, D-chiro-inositol and oligocyclitols(23). The outcome of the present study showed that Mucuna pruriens seed extract and Glibenclamide treatment significantly decreases the hyperglycemia caused by STZ.
Anusha et. al, reported that alcohol extract of Mucuna pruriens seeds ef- fectively reducing blood glucose level in Type I diabetic animal models and concluded that the hyperglycemic activities may be attributed to its rich dietary fibre content, which reduces glucose absorption , suggesting an extra pancreatic mechanism. Another interesting point we observed in this study was the efficacy of glibenclamide found to more than Mucuna Pruriens in the initial two weeks and towards the end of the treatment period Mucuna showed improved hypoglycemic effect.
This evidence suggests the efficacy of MP seeds in long term treatment I diabetes. Majekudonmi et al also put forwarded the same hypotheses in 12 weeks long animal study on type I diabetic rats. In the present study, analysis of lipid profile revealed decrease in the high density lipoproteins (HDL) and also Total Protein levels (TP) with subsequent increase in Low Density Lipoproteins (LDL), these evidences supports the observations made by Enechi et al. and Murugan et al., The proposed mechanism may be due to the presence of squalenes, which increases biliary cholesterol excretion, leads to decreased level of serum cholesterol (30). The animals treated with STZ are appeared ill-looking with reduction in body weight due to the injurious effects of STZ which caused alkylation of DNA and produced hyperglycemia and necrotic lesions. Present observations are in agreement with the findings of Mohammad Zafar et al (2010); Piyachaturawat et al .Weight gaining in the Mucuna Pruriens group was statistically significant when compared to STZ and Glibenclamide.
This may be attributed to the antioxidant property of the drug(24)(26). Studies conducted by Stephen. O. Majekodunmi et al (2011) also supporting the findings of our study. The structural changes seen in diabetic rats are due to DNA methylation activity and free radical production by STZ. It has been reported in various studies that increased oxidative stress may play a role in the pathogenesis and progression of diabetic tissue damage(8)(11)(12). Here, the partial destruction of the islet beta cells produced moderate hypoglycemia without ketosis in STZ injected animals (26)(28).
Moderate restoration in islet beta cell mass seen in Mucuna Pruriens group may be due to its rich antioxidant properties(27) . The structural changes observed in liver histology were the distorted hepatic lobule, necrosis, vacoulations in hepatocytes, fatty changes and lymphatic infiltrations in the parenchyma(29) (11). These changes indicate the metabolic disturbances generated by STZ through oxidative stress. Mucuna seed extract partially reversed liver pathology by controlling hepatocellular necrosis, preventing cellular infiltrations and vacoulations. Thus, the present study indicates Mucuna seeds possess hypoglycemic and hypolipidemic properties and reduces the lesions in Liver and Pancreas by diabetes.
CONCLUSION
Administration of Mucuna Pruriens extract alleviated the streptozotocin lesions in experimental rat group and ameliorated to certain extent in histopatholgical lesions and morphological alterations produced by Streptozotocin. Mucuna Pruriens may block many other complications of diabetes by reducing oxidative stress and hence protects from oxidative damage and dyslipidemia. It is recommended to conduct a long term study for a close evaluation of the anti diabetic properties of Mucuna pruriens seeds.
ACKNOWLEDGEMENTS
The author is thankful to Mr. Lokesh Maran A, Assistant professor in Statistics, Department of Community medicine, for his valuable suggestions and Mr. Chandrasekar, Histology lab technician, Department of Anatomy, for his assistance in histological preparations. We are thankful to the scholars whose articles are cited and included in references of this manuscript and we are grateful to authors, editors and publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed. Conflict of interest The authors don’t have any conflict of interest.

References:
1. Soumya D, Sreelatha S. Late Stage Complications of Diabetes and Insulin Resistance. J Diabetes Metab. 2011 Dec 25;2(9):PP 2:167.
2. Ahmed AM. History of diabetes mellitus. Saudi Med J. 2002 Apr;23(4):373-8.
3. Lakhtakia R. The History of Diabetes Mellitus. Sultan Qaboos Univ Med J. 2013 Aug;13(3):368-70.
4. Cerf ME. Beta Cell Dysfunction and Insulin Resistance. Front Endocrinol [Internet]. 2013
5. Ginter E, Simko V. Global prevalence and future of diabetes mellitus. Adv Exp Med Biol. 2012;771:35-41.
6. Teoh SL, Latiff AA, Das S. A histological study of the structural changes in the liver of streptozotocin-induced diabetic rats treated with or without Momordica charantia (bitter gourd). Clin Ter. 2009;160(4):283-6.
7. Shetty AK, Kumar GS, Sambaiah K, Salimath PV. Effect of bitter gourd (Momordica charantia) on glycaemic status in streptozotocin induced diabetic rats. Plant Foods Hum Nutr Dordr Neth. 2005 Sep;60(3):109-12.
8. Abdollahi M, Zuki ABZ, Goh YM, Rezaeizadeh A, Noordin MM. Effects of Momordica charantia on pancreatic histopathological changes associated with streptozotocin-induced diabetes in neonatal rats. Histol Histopathol. 2011 Jan;26(1):13-21.
9. Gupta RN, Pareek A, Suthar M, Rathore GS, Basniwal PK, Jain D. Study of glucose uptake activity of Helicteres isora Linn. fruits in L-6 cell lines. Int J Diabetes Dev Ctries. 2009;29(4):170-3.
10. F Waer H. Cytological and Histochemical Studies in Rat Liver and Pancreas during Progression of Streptozotocin Induced Diabetes and Possible Protection of Certain Natural Antioxidants. J Nutr Food Sci.Vol. 2(9); 2012: 112-125.
11. Jelodar GA, Maleki M, Motadayen MH, Sirus S. Effect of fenugreek, onion and garlic on blood glucose and histopathology of pancreas of alloxan-induced diabetic rats. Indian J Med Sci. 2005 Feb;59(2):64–69.
12. Majekodunmi SO, Oyagbemi AA, Umukoro S, Odeku OA. Evaluation of the anti-diabetic properties of Mucuna pruriens seed extract. Asian Pac J Trop Med. 2011 Aug;4(8):632-6.
13. Dalal PK, Tripathi A, Gupta SK. Vajikarana: Treatment of sexual dysfunctions based on Indian concepts. Indian J Psychiatry. 2013 Jan;55(Suppl 2):S273-6.
14. Muthu, Krishnamoorthy. Evaluation of androgenic activity of Mucuna pruriens in male rats. Afr J Biotechnol. 2011 Oct 26;10(66):15017-9.
15. Suresh S, Prithiviraj E, Lakshmi NV, Ganesh MK, Ganesh L, Prakash S. Effect of Mucuna pruriens (Linn.) on mitochondrial dysfunction and DNA damage in epididymal sperm of streptozotocin induced diabetic rat. J Ethnopharmacol. 2013 Jan 9;145(1):32-41.
16. Dart RC, editor. Medical toxicology. 3rd ed. Philadelphia: Lippincott, Williams and Wilkins; 2004. 1914 p.
17. Manyam BV, Dhanasekaran M, Hare TA. Neuroprotective effects of the antiparkinson drug Mucuna pruriens. Phytother Res. 2004 Sep;18(9):706-12.
18. Eleazu C, Eleazu K, Chukwuma S, Essien U. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J Diabetes Metab Disord. 2013;12(1):60.
19. Srinivasan K, Ramarao P. Animal models in type 2 diabetes research: an overview. Indian J Med Res. 2007 Mar;125(3):451- 72.
20. Aberare O, Okuonghae P, Mukoro N, Dirisu J, Osazuwa F, Odigie E, et al. Triglycerides, total cholesterol, high density lipoprotein cholesterol and low density lipoprotein cholesterol in rats exposed to premium motor spirit fumes. North Am J Med Sci. 2011;277-80.
21. Proks P, Reimann F, Green N, Gribble F, Ashcroft F. Sulfonylurea stimulation of insulin secretion. Diabetes. 2002 Dec;51 Suppl 3:S368–76.
22. Mountjoy KG, Finlay GJ, Holdaway IM. Effects of metformin and glibenclamide on insulin receptors in fibroblasts and tumor cells in vitro. J Endocrinol Invest. 1987 Dec;10(6):553-7.
23. Donati D, Pagani R, Guerranti R, Cinci G, Marinello E. Antidiabetic oligocyclitols in seeds of Mucuna pruriens. Phytother Res. Dec2005;19;(19(12))::1057-60.
24. Enechi, Ozougwu. Effects of ethanol extract of Mucuna pruriens leaves on the lipid profile and serum electrolytes of rats. 9(2):PP 18-23.
25. Bhaskar A, Vidhya VG, Ramya M. Hypoglycemic effect of Mucuna pruriens seed extract on normal and streptozotocin-diabetic rats. Fitoterapia. 2008 Dec;79(7-8):539-43.
27. Lampariello LR, Cortelazzo A, Guerranti R, Sticozzi C, Valacchi G. The Magic Velvet Bean of Mucuna pruriens. J Tradit Complement Med. 2012 Oct;2(4):331-9.
28. Bhaskar A, Nithya V, Vidhya VG. Phytochemical evaluation by GC-MS and anti hyperglycemic activity of Mucuna pruriens on Streptozotocininduced diabetes in rats. Journal of Chemical and Pharmaceutical Research 2011; 3(5): 689-696
29. Portha B, Blondel O, Serradas P. The rat models of non-insulin 10. dependent diabetes induced by neonatal streptozotocin. Diabet Metab1989; 15 : 61-75. 29.
30. Liu Y, Xu X, Bi D, Wang X, Zhang X, Dai H.Influence of squalene feeding on plasma leptin,testosterone and blood pressure in rats. Indian Journal of Medical Research 2009; 129: 150-153.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License