IJCRR - 13(3), February, 2021
Pages: 64-69
Date of Publication: 03-Feb-2021
Print Article
Download XML Download PDF
Pattern of Recurrence and Survival in TNBC - A Retrospective Study Done in a Rural Based Medical College and Hospital of West Bengal India
Author: Santanu Acharya, Krishnangshu Bhanja Choudhury, Partha Dasgupta, Atanu Basu, Pinaki Bhattacharyya, Sree Krishna Mandal
Category: Healthcare
Abstract:Introduction: Triple-Negative Breast Cancer (TNBC) constitutes patients with estrogen and progesterone hormone receptors negativity, Her 2-neu negativity and variable expressions of Ki67 (proliferative marker), EGFR and CK 5/6 cytokeratins. Standard treatment protocols have failed to improve the overall prognosis and DFS in TNBC, unlike other subtypes. Objective: Our retrospective study aims to identify the TNBC patients treated at rural tertiary medical college and determine their pattern of care and survival. Methods: A single institutional retrospective study was conducted on patients with TNBC subtype, registered at tertiary rural medical college, between May 2012 and April 2017. The study population included female patients, aged 15-70 years, with histology proven carcinoma of the breast, AJCC TNM 7th edition staging I to IV, hormone (ER and PR) and HER-2neu receptors negative, who had undergone surgery, chemotherapy and radiation (curative or palliative), either in neoadjuvant or adjuvant or palliative settings. Disease-Free Survival (DFS) and Overall Survival (OS) curves were calculated using the Kaplan- Meier method. Results: A total of 284 patients were eligible for study analysis. The median age was 45 years. 63.4% of patients presented with T3 or T4 tumour. Similarly, 165 (58.1%) patients presented with nodal disease. AJCC TNM stage II and III combined accounted for 90.1% of patients. 14 individuals had metastases at the time of diagnosis with lung, liver and bones being affected. The 5 years DFS in months was 63.3% with events occurring in 100 individuals. Conclusion: TNBC is an aggressive luminal breast carcinoma with advanced disease presentation, poor outcome to standard conventional chemotherapy and radiation resulting in reduced DFS and OS.
Keywords: Triple Negative Breast Cancer, HER2 mutation, Rural population
Full Text:
INTRODUCTION
Breast carcinoma classification has progressed a lot, from older pathological subtypes to receptor-based luminal and now to present micro DNA assay.1,2 According to luminal subtypes, major subgroups are luminal A, B, Her 2 neu enriched and Basal type, which includes Triple-Negative Breast Cancer (TNBC). TNBC constitutes patients with estrogen and progesterone hormone receptor negativity, Her 2-neu negativity and variable expressions of Ki67 (proliferative marker), EGFR and CK 5/6 cytokeratins.3 Unlike worldwide incidence of 15%, TNBC accounts for 31% (95% CI, 27% to 35%) of 7237 Indian patients in systemic review involving 17 studies.4,5 TNBC is mostly found among younger women less than 40 years or women with BRCA1 mutations. Despite being sensitive to chemotherapy, TNBC has a high propensity for early metastases to lung, brain and soft tissue. TNBC patients have a poorer prognosis compared with other molecular subtypes of breast cancer with short disease-free survival.6-10 Choice of chemotherapy has likewise evolved to overcome short disease-free survival from traditional anthracycline-taxane combination to platinum-based regimens to newer PTEN, PI3K pathway regulators.11 Suspected radioresistance in TNBC is probably related to more time available for repair of radiation-induced DNA damage in ER-negative cells.12 However radiation is still advocated on grounds of BRCA1 mutation and its lacking of double-stranded repair. So like the choice of chemotherapy, the role of postoperative radiation remains controversial.
The incidence of TNBC worldwide is around 15%. Indian scenario is somewhat highly variable. Sandhu et al. detected the incidence of TNBC as high as 31% among 7,237 Indian patients. In other Indian studies, TNBC incidence varied from 14% to 19.3%. Data on demographic profile, treatment and survival pattern among TNBC patients are limited from the Indian subcontinent, especially from patients of the rural population. Our retrospective study aims to identify the TNBC patients from rural population treated at rural tertiary medical college and determine their pattern of care and survival.
MATERIALS AND METHODS
A single institutional retrospective study was conducted on patients with TNBC subtype, registered at outpatients department at tertiary rural medical college, between May 2012 and April 2017. The study population included female patients, aged 15-70 years, with histology proven carcinoma of the breast, AJCC TNM 7th edition staging I to IV, hormone (ER and PR) and HER-2neu receptors negative, had undergone surgery, chemotherapy and radiation (curative or palliative), either in neoadjuvant or adjuvant or palliative settings. Patients who did not receive any treatment and had received treatment before registration (excepting surgery) at the department were excluded from the study.
Data was collected in Microsoft Office Excel spreadsheet Office 2007 after reviewing hospital records. The pattern of recurrence and survival in TNBC was determined using descriptive statistics. Disease-free survival (DFS) in months was defined as the time from completion of treatment of TNBC to first locoregional or distant metastasis or recurrence. Overall survival (OS) in months was defined as the time from diagnosis of TNBC (biopsy was taken) till death. DFS and OS curves were calculated using the Kaplan- Meier method.
RESULTS
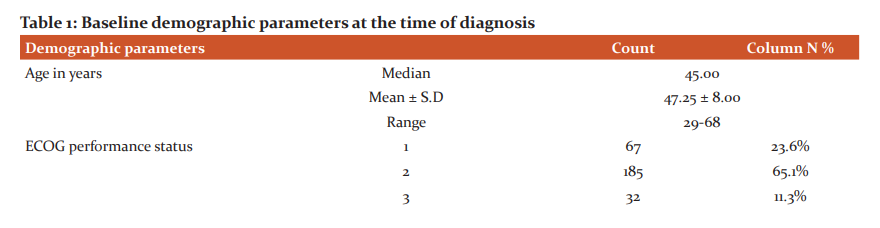
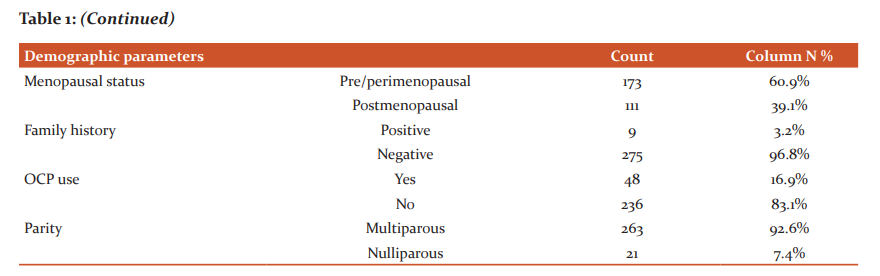
The retrospective single institutional study was conducted between 2012 and 2017. The total number of patients presenting as TNBC was 348, out of which 284 patients who had complete data retrieved from hospital medical records section were eligible for study analysis. The median age of our study population was 45 years, range 29-68 years (Table 1). Majority of patients were in ECOG performance status 2 (65.1%). 63.4% of patients presented with T3 or T4 tumour stage, which shows gross negligence and ignorance to seek treatment at early stages as well as the aggressiveness of the luminal subtype. Similarly, 165 (58.1%) patients presented with nodal disease. AJCC TNM stage II and III combined accounted for 90.1% of patients. At the time of diagnosis, 14 individuals had radiologically proven metastases with lung, liver and bones commonly being affected. None of the patients had synchronous or metachronous bilateral breast carcinoma over the study period.
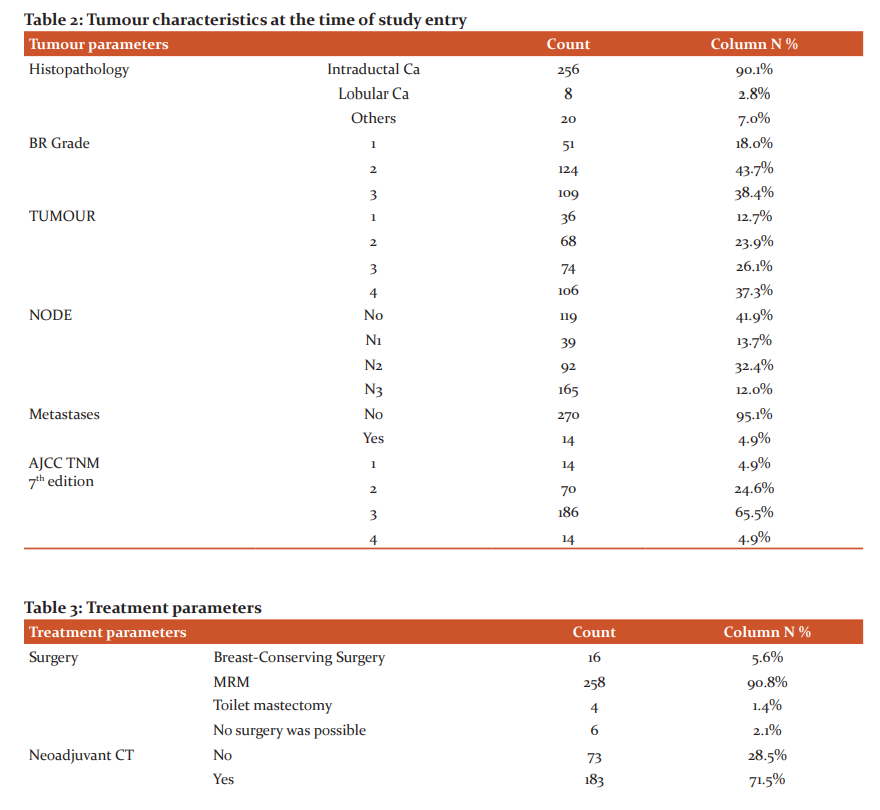
Breast-conserving surgery was possible in only 5.6% of individuals. Modified radical mastectomy was possible in 258 individuals. Toilet mastectomy was done in 4 patients with metastatic disease to relieve pain and bleeding. Neoadjuvant chemotherapy comprising of anthracyclines (doxorubicin – cyclophosphamide, 5fluorouracil – epirubicin – cyclophosphamide) combination was given to 27.3%, i.e 50 out of 183 patients who had received neoadjuvant chemotherapy. Sequential doxorubicin – cyclophosphamide followed by docetaxel or combination of Docetaxel - doxorubicin – cyclophosphamide regimens were used in 69.4% patients. Unlike 6 patients in the neoadjuvant setting, 30 patients in adjuvant settings received platinum doublets (docetaxel – carboplatin combination). Adjuvant radiation was administered in 247 individuals with usual dose regimens of 50Gray in 25fractions. For 14 patients who presented with metastatic disease upfront, palliative chemotherapy regimens used in curative settings were used. Palliative whole brain radiation (n=4) for brain metastases and radiation to bone metastases (n=10) were used (Table 3).
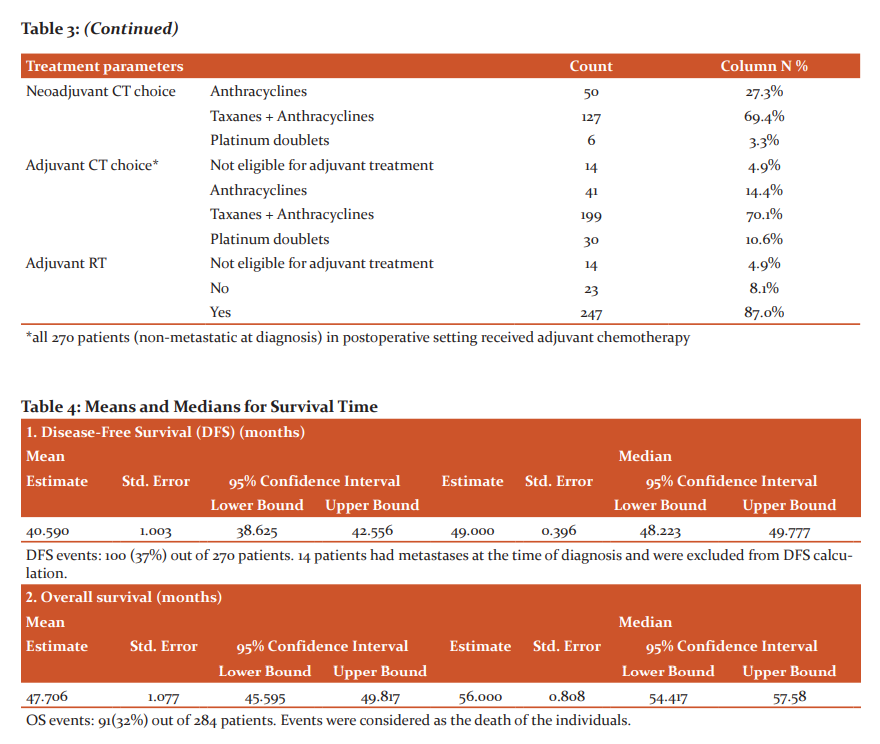
Median follow-up of all patients (n=284, at time of diagnosis) was 40 months (calculated by reverse Kaplan Meier Survival analysis). The 5 years DFS in months was 63.0% with events occurring in 100 individuals. 20 patients had an only local recurrence (chest wall recurrence in 5 patients, others lymph nodal involvement). 72 patients had distant metastases. Visceral involvement (lungs, liver, soft tissue, brain) in 50 patients, bones involvement only in 7 patients and combined visceral and bone in remaining. Both locoregional and distant recurrences/metastases occurred in 8 individuals. 5 years OS among 284 patients was 69% (table 4).
DISCUSSION
TNBC is a diverse luminal breast carcinoma, often considered a subtype of basal variety (75% similarity) characterized by absence of traditional ER, PR and Her-2-neu receptors and expression of Ki67, cytokeratin CK 5,6 and EGFR.1 This had rendered TNBC treatment equally controversial. With no receptors to be blocked, the choice of chemotherapy had ranged conventional taxane – anthracyclines to platinum doublets. Among all luminal subtypes, TNBC patients have increased incidence of distal metastases and early recurrences culminating in lower survival. The aggressiveness of TNBC has to lead to genetic studies to identify other possible pathways for carcinogenesis. The Cancer Genome Atlas (TCGA) Research Network using genomic DNA copy number arrays, DNA methylation, reverse phase protein arrays, exome sequencing, microRNA sequencing, and messenger RNA arrays, detected the loss of TP53, RB1 and BRCA1 genes in DNA damage repair mechanism, aberrant activations of phosphatidylinositol 3-kinase (PI3K) signalling pathways and activating mutations in PIK3CA.12-16 The heterogeneity in genetic configurations was also confirmed by Shah et al. who confirmed 12% of cases did not have the driver gene mutation.17 Lehman et al. detected 587 TNBC patients out of 3,247 breast cancer individuals using gene expression and classified TNBC into 6subtypes basal-like (BL1 and BL2), immunomodulatory (IM), mesenchymal (M), mesenchymal stem-like (MSL), and luminal androgen receptor (LAR).18 However, in 2015, Burstein et al. reclassified TNBC into 4 molecular subtypes: luminal-AR (LAR), mesenchymal (MES), basal-like immune-suppressed (BLIS), and basal-like immune-activated (BLIA).19
While advances have been made in the detection and management of TNBC based on newer DNA microassay, the situation in Indian perspective is still dismal. A meta-analysis was done by Sandhu et al. involving 7,237 Indian patients from 17 studies on breast cancer, diagnosed between January 1, 1999, and December 31, 2015. TNBC prevalence was 31% (95% CI, 27% to 35%) which was much higher than the western data.5 Overall prevalence of TNBC was high in the south (34%) and west (31%) of the country unlike in north (28%) and east (30%). The mean age of patients ranged from 43 to 55 years across studies, with a weighted average of age 50 years. The proportion of patients with grade 3 diseases ranged from 16% to75%, with a weighted average of 57%. There was substantial heterogeneity across the studies (I2 of 91.2% [95% CI, 88% to 94%], p-value 0.001).
The median age of our study population was 45 years, range 29-68 years. T3 and T4 disease were present in 63.4% of patients. Similarly, 165 (58.1%) patients presented with nodal disease. AJCC TNM stage II and III combined accounted for 90.1% of patients. Similar findings were reflected by Chintalapani et al. who studied TNBC patients in retrospective analysis between 2009 and 2014. A total of 1024 breast cancer patients, TNBC accounted for 198 (19.3%) of all breast cancers. The median age at the diagnosis was 50 years (range, 22–78 years). Lymph node involvement in TNBC variety was strongly associated with large tumour size (P = 0.003) and higher tumour grade (p = 0.01). The median follow-up was of 48 months (range, 12–88). The authors concluded that TNBC had high and early recurrences evident from 36 (19.1%) patients who had recurrences. Lost to follow up was pretty high at 14%. Visceral metastases were very common with lung recurrences in 52.7% individuals followed by bone (25%) and brain (11.1%). Three-year DFS and OS were 63.2% and 65.6%, respectively. Unlike univariate analysis where nodal status, size of the tumour, and lymphovascular invasion were found to have a significant impact on OS and DFS, only lymph nodal status was found to be significant for DFS and OS (p < 0.001 and p = 0.001, respectively) on multivariate analysis.20
In a 16 years long retrospective multicentric study (2002 to 2018), 100 patients out of 711 breast cancer individuals were diagnosed as TNBC. The median follows–up time was 11.2 years. TNBC was detected at the much younger median age of 34 years. The left breast was involved in 50% of patients with 2% having bilateral disease. Metastasis was present in 10% of the TNBCs with lungs as the most affected organ (7%)by bone (3%). Majority of the patients were in stages 2 and 3. 85% received adjuvant chemotherapy and 15% received neoadjuvant chemotherapy. Modified radical mastectomy was done in 80% of the patients. Breast-conserving surgery was done in 20%. 45% received radiotherapy. The relapse rate was 5% with 6% alive with disease and 77% alive with no recurrences.21 In our study de novo metastases was detected in 4.9% of patients. The 5 years DFS in our study was 63.0% with events occurring in 100 individuals. 20 patients had an only local recurrence (chest wall recurrence in 5 patients, others lymph nodal involvement) with 72 patients had distant metastases only and others (n=8) with both locoregional recurrences and distant metastases.
In a previous study from the same hospital, co-authored by us, recurrence pattern and survival among TNBC patients were analyzed from April 2010 to March 2015. The median age in 2010-2015 study was 47years (range 22-72 yr). DFS at 3 yrs was 64.43% and OS was 75.73% with 35.14% patients developing disease progression during the first 3 yrs after primary treatment. 5 yr DFS was 61.08% and OS was 71.54%.22 In our present study we had analysed 284 patients out of 348 TNBC patients. The analyzed sample population had increased from 239 in 2010-2015 to 2012-2017 study. This reflects higher pathological testing of receptors of biopsy specimens. Locally advanced disease was prevalent during both study periods, which shows ignorance persisting in our rural population in seeking early advice.
Our study has several limitations like it’s observational single institutional retrospective design, no separate markers like cytokeratin EGFR, CK5/6 or Ki67 were done in TNBC patients and many patients had sampling error where receptor analysis could not be done to ascertain the luminal subtypes.
Conclusion
TNBC is an aggressive heterogeneous luminal breast carcinoma with the poor outcome to standard conventional chemotherapy and radiation resulting in reduced DFS and OS. Whether modern targeted drugs linked to other markers like CK5/6 and EGFR improves the survival needs to be studied in future studies.
Acknowledgement: Authors acknowledge the immense help received from the scholars whose articles are cited and included in references to this manuscript. The authors are also grateful to authors/editors/publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
-
Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature 2000;406:747–752.
-
Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 2004;10:5367–5374.
-
Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007;13:4429-4434.
-
Swain S. Triple-Negative Breast Cancer: Metastatic Risk and Role of Platinum Agents 2008 ASCO Clinical Science Symposium, 2008. June 3, 2008.
-
Sandhu GS, Erqou S, Patterson H, Mathew A. Prevalence of Triple-Negative Breast Cancer in India: Systematic Review and Meta-Analysis. J Glob Oncol 2016;2:412?421.
-
Hamm C, El-Masri M, Poliquin G, Poliquin V, Mathews J, Kanjeekal S, et al. A single-centre chart review exploring the adjusted association between breast cancer phenotype and prognosis. Curr Oncol 2011;18:191-196.
-
Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, et al. The triple negative paradox: primary tumour chemosensitivity of breast cancer subtypes. Clin Cancer Res 2007;13:2329–2334.
-
Liedke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 2008;26:1275–1281.
-
Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer. Cancer 2008;113:1638–1645.
-
Von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M et.al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol 2014;15:747–756.
-
Langlands FE, Horgan K, Dodwell DD, Smith L. Breast cancer subtypes: response to radiotherapy and potential radiosensitisation. Br J Radiol 2013;86(1023):20120601.
-
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. Nature 2012;490:61-70.
-
Andre F, Job B, Dessen P, Tordai A, Michiels S, Liedtke C, et al. Molecular characterization of breast cancer with high-resolution oligonucleotide comparative genomic hybridization array. Clin Cancer Res 2009;15:441-451.
-
Gewinner C, Wang ZC, Richardson A, Teruya-Feldstein J, Etemadmoghadam D, Bowtell D, et al. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signalling. Cancer Cell 2009;16:115-125.
-
Saal LH, Holm K, Maurer M, Memeo L, Su T, Wang X, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res 2005;65:2554-2559.
-
Abramson VG, Lehmann BD, Ballinger TJ, Pietenpol JA. Subtyping of triple-negative breast cancer: implications for therapy. Cancer 2015;121:8-16.
-
Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 2012;486:395-399.
-
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011;121:2750-2767.
-
Burstein MD, Tsimelzon A, Poage GM, Covington KR, Contreras A, Fuqua SA, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res 2015;21:1688-1698.
-
Chintalapani SR, Bala S, Konatam ML, Gundeti S, Kuruva SP, Hui M. Triple-negative breast cancer: Pattern of recurrence and survival outcomes. Ind J Med Paediatr Oncol 2019;40:67-72.
-
Rajendran T, Prasad K. Clinical profile of triple-negative breast cancer in Indian women: Long term follow-up study. J Clin Oncol 2018;36(15):e13109-e13109.
-
Mandal R, Acharyya S, Mollah Md A, Ghosh A, Pal A.C. Analysis of Patterns of Recurrence & Survival In Triple-Negative Breast Cancer Patients In A Rural Based Medical College Hospital of West Bengal, India : A Retrospective Study. J Dent Med Sci 2017;16:36-41.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License