IJCRR - 13(3), February, 2021
Pages: 60-63
Date of Publication: 03-Feb-2021
Print Article
Download XML Download PDF
Antibacterial Effects of ZnO NPs Synthesized by Solvothermal Method against Pseudomonas sp. Extracted from Saliva Ejector Tubing
Author: Sharmili Pisal, Jayant Pawar, S. C. Kale, Jayashri Nanaware, Rabinder Henry, Shilpa Ruikar, Snehal Masurkar
Category: Healthcare
Abstract:Introduction: The widespread antibacterial activity of nanoparticles of zinc oxide (ZnO-NPs), specifically by using nano-technology to synthesize its nanoparticles, has had considerable attention. Objective: In the present study synthesis of ZnO-NPs by the solvothermal method and its antibacterial efficacy against Pseudomonas sp. has to be studied. Methods: The precursor 0.1M zinc sulfate and reducing agent 0.1M NaOH were used for the synthesis of ZnO-NPs. The synthesized ZnO-NPs were additionally, characterized biochemically using UV-vis Spectroscopy and X-ray diffraction (XRD) analysis. Results: The λmax and band gap was found to be at 329 nm and 3.87 eV respectively. Bacteria found in contact oral cavities forms biofilms which makes them resistant to orthodox antimicrobial agents. Therefore, to overcome this problem, the present work was focused on the alternative approach based on nanomaterials. Antibacterial efficacy of ZnO NPs was performed against Pseudomonas sp. by Methylene blue reduction assay. Significant inhibition was observed (17 mm) at a 100 µg/mL concentration of ZnO-NPs. Conclusion: Reactive oxygen species (e.g. H2 O2 ) was a critical influence in many events, including the lack of proton motives as well as the harmful absorption of dissolved toxic Zinc ions as a result including cell wall trauma, increased membrane permittivity, excessive uptakes of dissolved ions of Zn, etc. This contributed to mitochondrial weakening, intra-cellular discharge as well as the release of oxidative stress within the expression of genes, that resulted in inhibition of cell proliferation and cell death.
Keywords: Antibacterial, Solvo-thermal Method, Oral cavities, Biofilm, Biochemical characterization, Saliva ejector tubing
Full Text:
INTRODUCTION
The most widely recognized clinical illness affecting immuno-compromised deceitful people is P. aeruginosa. The treatment of infectious diseases has become an emerging problem due to multi-drug resistance. Wide-ranged usage for several human bacterial infections with antibiotics of the broad spectrum also culminated in resistance to antibiotics. Advanced nano-medicine insights have been opened up recently in the area of nano-technology, allowing for the production of nano-particles.1 The new class of Antimicrobial Agents which are extremely being researched for their anti-bacterial properties, as well as its potential applications in food, health care and the environmental sustainability, is the metal oxides nano-particles like zinc oxide (ZnO), magnesium oxide (MgO), cupric oxide (CuO), calcium oxide (CaO), silver oxide (Ag2O).2-6
MATERIALS AND METHODS
Isolation and Biochemical Characterization
Bacterial isolates were obtained from swabs collected from the oral cavity. The isolates were maintained on Mannitol Salt Agar medium (MSA). Random colonies were selected and their colony characteristics were recorded. Microscopic examination including Gram staining was performed. Biochemical characterization of the isolates was performed by Voges Proskauer, indole, catalase and methyl red tests.
Synthesis and Characterization of Zinc Oxide Nanoparticles (ZnO-NPS)
Zinc sulfate (0.1M) was dissolved in 50ml distilled water by continuous stirring on the magnetic stirrer. 0.1M NaOH solution (100 ml) was dropwise added into the reaction mixture at 90 °C for 2 hours. The reaction mixture was centrifuged at 5000 rpm for 15 minutes. The pellet was washed three times with distilled water and air-dried at 60 °C for 24 hours, followed by calcination at 400 °C for 4 hours. The resultant precipitate was characterized by UV-Vis Spectroscopy and X-Ray Diffraction (XRD) analysis to obtain optical and structural information of the material.
Screening of biofilm-forming isolates and tube check reduction using Red Agar Congo Test
The bacterial isolate was inoculated on Congo red agar medium and incubated at 37°C ± 2°C for 24 hours. Biofilm production was investigated by observing dry crystalline black colonies on congo red agar plate.7
Tube Assay
The isolate was inoculated for 24 hours in tubes containing Trypticase Soy broth. The assay was disposed of and the sampled tubes with a phosphate buffer saline (PBS) were cleaned post-incubation. After that, 0.1% crystal violet was darkened for 5 minutes in the tubes as well as the deionized water poured overdye. Purple colour on the tube wall suggests biofilm development.8
Determining the Antibacterial activity of ZnO NP action against Pseudomonas sp. Test of Blue Methylene Removal (MBRT)
For qualitative determination of cell viability of metabolically active Pseudomonas sp. cells on the exposure of ZnO NPs, methylene blue reduction test (MBRT) was performed6. Briefly, Pseudomonas sp. 1mL culture broth was blended with 1x105 CFU/mL mixed into 0.1mL (0.5%) methylene blue dye. As a consequence, a tube of 0.5mL of ZnO NPs (1mg/mL), as well as positive (containing bacterial culture and MB) including negative control (containing nutrients broth and MB) is applied as well as incubated in complete darkness under 37°C±2°C for 4 hours. All experiments were performed in triplicates.
The purpose of this experiment was to qualitatively determine cell viability of metabolically active B. cereus cells by MBRT in presence of CuO NPs. The antibacterial e?cacy of CuO NPs was determined by MBRT. Brie?y, 1 mL of selected bacterial culture (B. cereus)of1×105 CFU/mL inoculated in the nutrient broth was mixed with 0.1 mL of 0.5% methylene blue in two Eppendorf tubes. Subsequently, in one tube 0.5 mL of CuO NPs (1 mg/mL) was added and kept for incubation at 37°C ± 2°C in the incubator for 4 h along with positive (containing bacterial culture and MB) and negative control (containing nutrient broth and MB). All reaction tubes were covered in silver foil and kept for incubation under dark conditions in closed incubator. All experiments were performed
in triplicates. The purpose of this experiment was to qualitatively determine the cell viability of metabolically active B. cereus cells by MBRT in presence of CuO NPs. The antibacterial e?cacy of CuO NPs was determined by MBRT. Brie?y, 1 mL of selected bacterial culture (B. cereus)of1×105 CFU/mL inoculated in the nutrient broth was mixed with 0.1 mL of 0.5% methylene blue in two Eppendorf tubes. Subsequently, in one tube 0.5 mL of CuO NPs (1 mg/mL) was added and kept for incubation at 37°C ± 2°C in the incubator for 4 h along with positive (containing bacterial culture and MB) and negative control (containing nutrient broth and MB). All reaction tubes were covered in silver foil and kept for incubation under dark conditions in closed incubator. All experiments were performed
in triplicates. The purpose of this experiment was to qualitatively determine the cell viability of metabolically active B. cereus cells by MBRT in presence of CuO NPs. The antibacterial e?cacy of CuO NPs was determined by MBRT. Brie?y, 1 mL of selected bacterial culture (B. cereus)of1×105 CFU/mL inoculated in the nutrient broth was mixed with 0.1 mL of 0.5% methylene blue in two Eppendorf tubes. Subsequently, in one tube 0.5 mL of CuO NPs (1 mg/mL) was added and kept for incubation at 37°C ± 2°C in the incubator for 4 h along with positive (containing bacterial culture and MB) and negative control (containing nutrient broth and MB). All reaction tubes were covered in silver foil and kept for incubation under dark conditions in closed incubator. All experiments were performed in triplicates.
RESULTS AND DISCUSSION
Isolation and Biochemical Characterization
All the isolates were found to be motile Gram-negative rods. Isolate found catalase-positive gives a negative methyl red, indole and Voges Proskauer tests, but a positive catalase test. Therefore, it was tentatively identified as Pseudomonas sp.
Characterization of ZnO-NPs
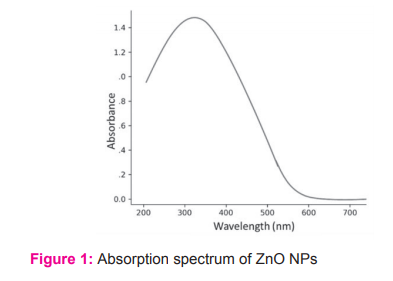
ZnO-NPs were synthesized using solvothermal method. Figure 1 shows the absorption curve for Zn Opower. The absorption spectrum and bandgap of synthesized powder were found to be at 329 nm and 3.87 eV respectively.
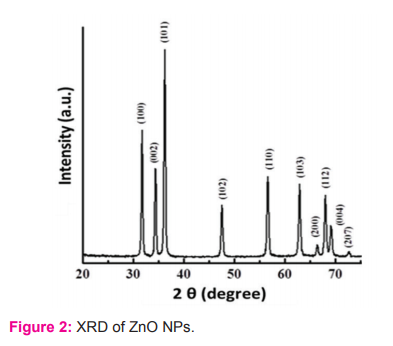
About the Joint Committee on Powder Diffraction Standards (JCPDS) for XRD, card number 36-1451, the diffractogram of ZnO powder shows the creation of hexane crystal structure. The extension of the top demonstrates the creation of nano-scale structures. The study of powders reveals crystalline nature at peaks (100), (002), (101), (102), (110), (103), (112) and (207) with a similar degree of crystalline nature (Figure 2). To observe synthesised ZnO-NPs, the UV-visible-spectroscopy was sonicated for around 15mins in distilled water, the UV spectrum was then noted. The amplitude of the peak absorption in the UV range is stated to be correlated to nano-particles' size. Therefore peak moves to a lower wavelength (blue shift) on the decrease of the particle's sizes.
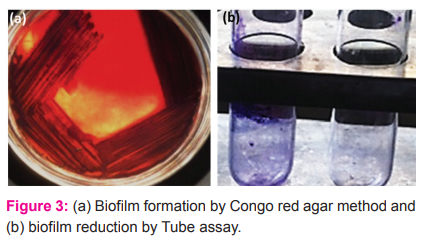
Screening of isolate for biofilm formation and reduction after the exposure of ZnO NPs:
Isolated Pseudomonas sp. were screened for biofilm formation. After 48 hours, black colonies on congo red agar medium indicated their biofilm-forming ability. The reduction of biofilm was determined by the visual appearance of the fragmented violet colour layer on the wall of the test tube through tube assay (Figure 3).
The MBRT is focused on the assumption that the colour on a medium provided by the application of MBRT is diminished with bacteria as well as the colour decolouration rates by the amount of metabolically active bacterial cells present in it is in inverse proportion to the rate of oxygen consumed.6 Pseudomonas sp. strength. The MBRT has been calculated for ZnO NPs, which relies on the degree to which they live bacterial cells as well as the levels of oxygen in tube decoloratemethyle blue. This research deals on the concept of colour decrease and induces the decolouration of the bacterial cell membrane by reductase enzyme.
The research has shown a total reduction of the incorporated dye after 4 hours culturing in the MB dye in bacterial suspension, which includes only bacterial cells and has shown that the live cells were capable of dye reductions. In experiments involving nutrient broth, MB, bacterial cells and ZnO NPs, a drop in dye was not found, demonstrating specifically, that ZnO NPs destroy bacteria in the study such that the nutrient oxygen will not use the bacteria as a material that decreases the volume of toxicity. Thus, ZnO NPs have demonstrated against Pseudomonas sp. antibacterial activity (Figure 4). In negative tests including the nutrient broth and MB, though, no dye reduction had been seen. The degree of discolouration was found to be directly related to the viability cells.

Figure 4: Pseudomonas sp. anti bacterium ZnO NPs activity. he non-coloured tube contains reduced MB, metabolically-active bacterial cells, as well as the coloured tube contained metabolically inactive bacterial cells that were eliminated from ZnO NPs action, before MBRT (a) and post 4-hr incubation (b).
CONCLUSION
It is necessary to acquire important information about the behaviour of ZnO NPs and their antibacterial effects in the biological fluid, namely saliva, which faithfully reflects the oral cavity environment. We have shown that nano-particles can be regulated by such methods in their shape and scale. Exploring XRD shows the integrity of the process. The crystallite form of the synthesized nano-particles is hexagonal roots composition. UV spectroscopy shows that the borders of emission are moved to a lower wavelength with a blue change owing to a reduction in crystal size. The DEG and TEG molecule adsorbed in nanoparticles ZnO were provided by FTIR and TGA research. Both ZnOnano-particles are formulated and have antibacterial as well as antibiotic action against Pseudomonas sp. Colloidal ZnO-NPs were most efficient in eradicating all the tested oral bacteria. The antimicrobial activity including antibiofilm potency of ZnO NPs has improved as the particle size has reduced. The use of nanoparticles as antimicrobials in the treatment of oral diseases, however, is becoming increasingly significant in terms of their biocidal, anti-bacterial, antiadhesive and transmission capability. They are currently being researched as components of prothesized metal coatings, topical delivery agents including orthodontic applications. In the future, such nanoparticles with the highest antibacterial activities as well as having minimum toxic effects host are expected to be produced.
ETHICAL ISSUE: Ethical clearance was taken from institutional ethical committee, KIMSDU, Karad, Maharashtra.
FUNDING SOURCES: Krishna Institute of Medical Sciences Deemed To Be University, Karad.
CONFLICT OF INTEREST: Nil
ACKNOWLEDGEMENTS: We are grateful for the support which we received from the TERA Research Institute India Pvt. Ltd., Pune, Maharashtra, India, Directorate of Research, Krishna Institute of Medical Sciences “Deemed to be University, Karad, Maharashtra, India, Department of Allied Sciences, Krishna Institute of Medical Sciences “Deemed to be University, Karad, Maharashtra, India.
References:
-
Franci G, Falanga A, Galdiero S, Palomba L, Rai M, Morelli G, et al. Silver nanoparticles as potential antibacterial agents. Molecules 2015;20(5):8856-8874.
-
Stoimenov PK, Klinger RL, Marchin GL, Klabunde KJ. Metal oxide nanoparticles as bactericidal agents. Langmuir 2002;18(17):6679-6686.
-
Jin T, He Y. Antibacterial activities of magnesium oxide (MgO) nanoparticles against foodborne pathogens. J Nanopart Res 2011;13(12):6877-6885.
-
Pawar J, Shinde M, Chaudhari R, Singh EA. Semi-solvothermal synthesis and characterization of zinc oxide nanostructures against foodborne pathogens. Int J Pharm Bio Sci 2017;8(2):311-315.
-
Pawar J, Prakash V, Vinchurka P, Shinde M, Henry R, Mandke Y, et al. Application of Semi-Solvo Thermally Synthesized Zinc Oxide (ZnO) Nanoparticles in Food Technology and Its Characterization. Int J Nanotechnol Appl 2017;11(1):75-80.
-
Pawar J, Henry R, Viswanathan P, Patwardhan A, Singh EA. Testing of antibacterial efficacy of CuO nanoparticles by methylene blue reduction test against Bacillus cereus responsible for food spoilage and poisoning. Ind Chem Engg 2019;61(3):248-253.
-
Freeman DJ, Falkiner FR, Keane CT. New method for detecting slime production by coagulase negative staphylococci. J Clin Path 1989;42(8):872-874.
-
Christensen GD, Simpson WA, Bisno AL, Beachey EH. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun 1982;37(1):318-326.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License