IJCRR - 13(3), February, 2021
Pages: 06-10
Date of Publication: 03-Feb-2021
Print Article
Download XML Download PDF
Hepatoprotective Activity of Different Extract of Chromolaena Odorata Against CCL4 and Rifampicin\? Induced Hepatic Injuries in Rats: A Randomized Controlled Preclinical Trial
Author: Muthu Ramu T, Rajasekaran S
Category: Healthcare
Abstract:Introduction: Now a day's liver disease is a worldwide problem. Modern synthetic drugs used in the treatment of liver diseases are sometimes inadequate and can cause serious side effects. Objective: This current investigation aims to evaluate the ethanol and aqueous extracts of leaves of Chromolaena odorata (L.) R. M. King & H. Rob. (Asteraceae) was investigated against carbon tetrachloride and rifampicin-induced hepatic damage. The plant of Chromolaena odorata is a perennial herb and distributed throughout the region of India, Mexico and Asia. In traditionally this plant is used in the treatment of coughs, colds, and skin diseases. Based on the present study, an effort has been made to establish the scientific validity of the hepatoprotective activity against ccl4 and rifampicin-induced hepatotoxicity. Methods: To evaluate the hepatoprotective activity by various inducing agents like carbon tetrachloride at the rate of 1 ml/kg and rifampicin 1mg/kg produced liver damage in rats. Results: The results are manifested by the significant (P< 0.005) rise in serum levels of Serum oxaloacetate transaminase(SGOT), Serum glutamate pyruvate transaminase(SGPT), alkaline phosphatase (ALP), Total protein Totalbilirubin and Directbilirubin compared to respective control values. Histopathological observation also revealed that pretreatment with Chromolaena odorata protected the animals from carbon tetrachloride and rifampicin induced liver damage. Conclusion: From the present work we can strongly conclude that plant of Chromolaena odorata is biologically huge potential value. The preliminary chemical test of the samples revealed the presences of high-value phenolic compounds as the major content in the plant of Chromolaena odorata.
Keywords: Chromolaena odorata, CCl4, Rifampicin, Transaminases, Histopathological studies
Full Text:
INTRODUCTION
India is known for its traditional medicinal systems—Ayurveda, Siddha, and Unani. Medical systems are found mentioned even in the ancient Vedas and other scriptures. The Ayurvedic concept appeared and developed between 2500 and 500 BC in India.1,2 In India medicinal plants and traditional medicine have been the basis of traditional healthcare especially in remote areas where modern healthcare facilities are inadequate. India is the largest producer of medicinal plants is rightly called the “The botanical garden of the world”.3 The revival of interest in herbal medicines is due to safe, more accessible and more affordable. The plant of Chromolaena odorata (L.) R. M. King & H. Rob. family of Asteraceae is a perennial herb and distributed throughout the region of India, Mexico and Asia.4,5 In traditionally this plant is used in the treatment of coughs, colds, and skin diseases.6 Now a day’s liver disease is a worldwide problem. Modern synthetic drugs used in the treatment of liver diseases are sometimes inadequate and can cause serious side effects.7 On the basis of the present study, an effort has been made to establish the scientific validity of the hepatoprotective activity against CCl4 and rifampicin-induced hepatotoxicity.
MATERIALS AND METHODS
Plant Material
Fresh leaves of Chromolaena odorata.Were collected from kaaripatti, Salem (Dt) Tamilnadu. The plant was then authenticated by the Botanist A. Balasubramanium, consultant-central Siddha Research, Salem-Tamilnadu.
Preparation of the extract/drug
The fresh leaves of Chromolaena odorata dried at under shade, grind into small pieces of crude drugs with help of a mechanical grinder. The crude powder was passed through sieve no.30 and stored in a suitable container. Then the crude drugs were defatted with Ethanol 95% (75-78oC) by using soxhlet apparatus. Then crude drugs were then subjected to cold maceration method by using aqueous water for 72hrs. Then extracts were concentrated by using a rotary vacuum evaporator and kept aside in the desiccators. The extracts were suspended in Tween80 for the presented study. The extract obtained was subjected to various Preliminary Phytochemical Screening tests as per the procedure mentioned in the standard reference books.8,9 The extract was used for pharmacological evaluation
Preliminary Phytochemical Screening
The various extracts of Chromolaena odorata were then subjected to preliminary phytochemical analysis to assess the presence of various phytoconstituents, it revealed that the presence of alkaloids, steroids, polyphenolic constituents like flavonoids, saponins, glycosides, tannins, gums and mucilages.
Procurement of experimental animals
Swiss albino mice (20-25gm) and Wister rats (150-200gm) of either sex and approximate same age used in the present studies were procured from listed suppliers of Sri Venkateswara enterprises, Bangaloru, India. The animals were fed with standard pellet diet (Hindustan lever Ltd. Bangaloru) and water ad libitum. All the animals were housed in polypropylene cages. The animals were kept under the alternate cycle of 12 hours of darkness and light. The animals were acclimatized to the laboratory condition for 1 week before starting the experiment. The animals were fasted for at least 12 hours before the onset of each activity. The experimental protocols were approved by the Institutional Animal Ethics Committee (IAEC No.-60/2019/IAEC/VMCP.) after scrutinization. The animals received the drug treatment by oral gavage tube.
Acute toxicity test10
The ethanol and aqueous extract of Chromolaena odorata were subjected for acute toxicity, as per standard method (OECD/OCDE No: 423). Albino female mice weighing 20-28 gm were used in this present study. The animals were fed with standard pellet diet and water ad libitum. All the animals were housed in polypropylene cages. The animals were kept under the alternate cycle of 12 hours of darkness and light. The animals were acclimatized to the laboratory conditions for 1 week before starting the experiment. The dose of ethanol and aqueous extracts was prepared with suitable saline and was administered by intubations. The acute toxicity was screened up to doses of 5000mg/kg.
Hepatoprotective studies
Carbon tetrachloride [CCl4] induced hepatotoxicity in rat model11,12
The rats were divided into 7 groups of six animals in each. Group, I served as a control, Group II administered with carbon tetrachloride (1 ml/kg) in 50% v/v olive oil every 72 hrs, Group III was pretreated with the standard drug of silymarin (100mg/kg) orally for 7 days. Simultaneously CCl4 (1ml/kg) in 50% v/v olive oil every 72 hrs Groups IV, V animals were pretreated with Ethanol extract of Chromolaena odorata (200mg/kg, 400mg/kg respectively) for 7 days. Groups VI, VII animals were pretreated with aqueous extract of Chromolaena odorata (200mg/kg, 400mg/kg respectively) for 7 days and carbon tetrachloride (1 ml/kg) intraperitoneal every72 hours for 7 days for test groups. After the experimental period, blood was collected by the retro orbital method and the serum was separated and used for the assay of Serum oxaloacetate transaminase (SGOT), Serum glutamate pyruvate transaminase(SGPT), alkaline phosphatase (ALP), total protein, total &direct bilirubin were estimated respectively. The rats were sacrificed by cervical dislocation method. The liver is removed for histopathological examination
Rifampicin induced model13,14
The rats were divided into 7 groups of six animals in each. Group I served as a control, Group II administered with rifampicin (1gm/kg, p.o) every 72 hrs, Group III were pretreated with the standard drug of silymarin (100mg/kg) orally for 10 days. Simultaneously rifampicin (1 gm/kg) every 72 hrs, Groups IV, V animals were pretreated with Ethanol extract of Chromolaena odorata (200 mg/kg, 400mg/kg respectively) for 10 days. Groups VI, VII animals were pretreated with aqueous extract of Chromolaena odorata (200mg/kg, 400mg/kg respectively) for 10 days and rifampicin (1gm/kg) intraperitoneal every72 hours for 10 days for test groups. After the experimental period, blood was collected by the retro-orbital method and the serum was separated and used for the assay of Serum oxaloacetate transaminase (SGOT), Serum glutamate pyruvate transaminase(SGPT), alkaline phosphatase (ALP), total protein, total &direct bilirubin were estimated respectively. The rats were sacrificed by cervical dislocation method. The liver is removed for histopathological examination.
Statistical analysis
The values Mean± standard error means [SEM] are calculated for each parameter. For determining the significant intergroup difference each parameter was analysed separately and one-way analysis of variance was carried out and the individual comparisons of the group mean values were done using Dunnet’s test.15
RESULTS
Preliminary phytochemical screening revealed the presence of Alkaloids, Steroids, polyphenolic constituents like flavonoids, Saponins, glycosides, tannins, gums and mucilages. Acute toxicity studies of the various extracts of the Chromolaena odorata did not exhibit any signs of toxicity up to 5 g/kg body weight. Since there was no mortality of the animals found at the high dose. Hence 200 and 400 mg/kg dose of the extract selected for evaluation of the hepatoprotective activity.
CCl4 induced hepatotoxicity
A significant reduction was (P<0.05) observed in SGOT, SGPT, ALP, total bilirubin, direct bilirubin, increase in the total protein levels in the groups treated with 200& 400 mg/kg dose of Chromolaena odorata(L)king&H.Rob.extracts. The enzyme levels were almost to the normal. It was observed that the size of the liver was pale reddish-brown and enlarged in CCl4 intoxicants rat but it was normal in drug-treated groups. A significant reduction (P< 0.05) in liver enzyme level finding table no-1
Rifampicin induced hepatotoxicity
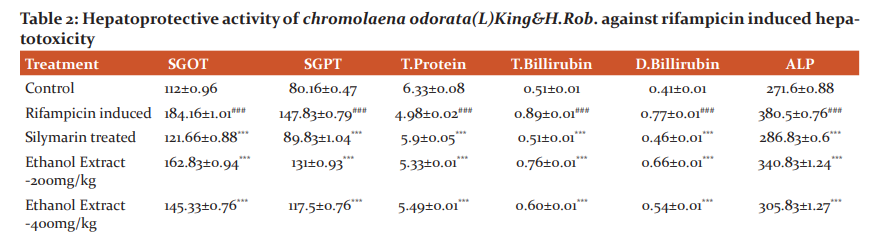
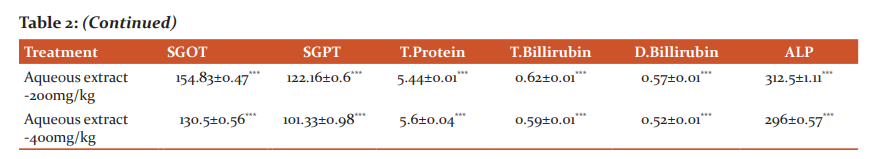
Table 1 summarizes the results obtained in the experimental model of rifampicin-induced hepatotoxicity in rats. The present study was subjected that aqueous extract is indicating dose-dependent hepatoprotective activity while the comparison to ethanolic extract against liver injury induced by rifampicin. A significant reduction (P< 0.05) in liver enzyme level finding table no-2
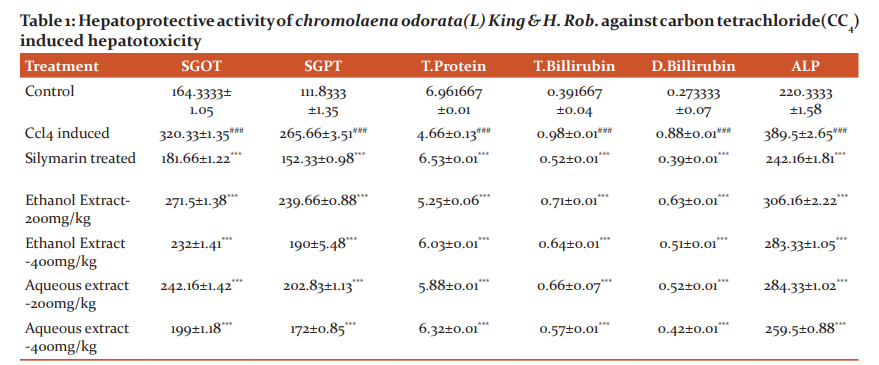
- Values are expressed as mean ± S.E.M. (n=6),
- #, normal control group compare with ccl4 treated group
- ### = p< 0.001,**=p< 0.01,*=p< 0.05
- *, compare with ccl4 treated group
- ***= p< 0.001, ## = p<0.01, # = p< 0.05
-
Values are expressed as mean ± S.E.M. (n=6),
-
#, normal control group compare with ccl4 treated group
-
### = p< 0.001,**=p< 0.01,*=p< 0.05
-
*, compare with ccl4 treated group
-
***= p< 0.001, ## = p<0.01, # = p< 0.05
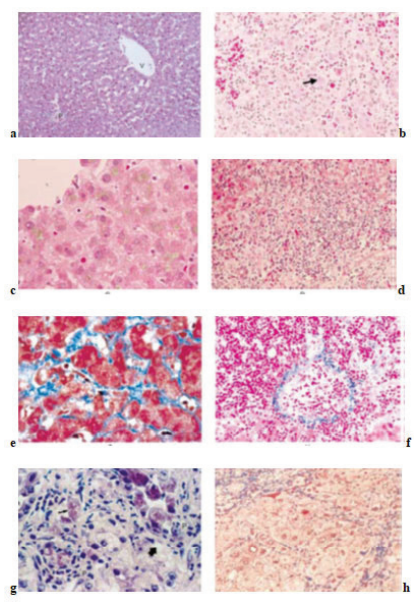
Figure 1: (a) liver of normal control rat; (b) Negative control (CCl4 iinuced); (c) standard drug treated; (CCl4 model); (d) standard drug treated (Rifampicin model) (e) Effect of ethanolic extract on CCl4 induced hepatotoxicity in rat; (f) Effect of aqueous extract on CCl4 induced hepatotoxicity in rat; (g) Effect of ethanolic extract on rifampicin induced hepatotoxicity in rat; (h) Effect of aqueous extract on rifampicin induced hepatotoxicity in rat.
DISCUSSION
Literature review revealed that various chemical and biological investigations were carried out with these plants. Phytochemical screening revealed the presence of Alkaloids, Steroids, polyphenolic constituents like flavonoids, Saponins, glycosides, tannins, gums and mucilages. Carbon tetrachloride (CCl4) is potent hepatotoxin producing centrilobular hepatic necrosis. It is accumulated in hepatic parenchyma cells and metabolized to CCl3 by liver Cytochrome P450-dependent monooxygenase its enzymatic transformation by CYP2E1 into a highly reactive free radical (CCl3•) and subsequent derivatives (Cl3COO) Those free radicals initiate and promote the propagations of lipid peroxidation.16,17 Administration of carbon tetrachloride showed significantly elevated levels of, SGOT, SGPT, ALP, TP and TB, due to its enzymatic activation of CCl3 free radical, which in turn alters the structure and function of liver cells. Treatment with aqueous extract of Chromolaena odorata(L) king & H.Rob. showed dose-dependent protection against the injurious effects of CCl4 when compared to ethanol extract. Rifampin (also referred to as rifampicin) is a macrocyclic antibiotic with major activity against mycobacteria, commonly used in combination with other agents as therapy of tuberculosis. Rifampicin extensively metabolized by the liver and induces multiple hepatic enzymes including Cytochrome [CYP 3A4 and Adenosine binding cassette C2 [CYP 3A4 and ABC C2], Multidrug resistance-associated protein-2 [MRP2]. Thus, the cause of injury is likely to be due to idiosyncratic metabolic products that are either directly toxic or induce an immunologic reaction. The rise in direct and total bilirubin in rare patients receiving rifampin may relate to gene defects in [MRP2, ABC C2] The major bilirubin glucuronide transporter in hepatocytes that is known to be abnormal in the Dubin-Johnson syndrome. Patients with pre-existing liver disease and cirrhosis are particularly likely to develop jaundice on rifampin therapy.18,19
CONCLUSION
The present study concludes that the ethanol and aqueous extracts of the leaves of Chromolaena odorata possesses significant hepatoprotective activity in Carbon tetrachloride (CCl4) induced rats at a dose of 1 ml/kg/i.p and rifampicin at a dose of 1mg/kg. Thus, it has been scientifically proved that the traditional knowledge obtained from the tribal people of South India is true and the extracts have enough potential as a hepatoprotective agent and hence worth investigative. Further study needs to be isolated, identify the active compounds and formulation.
ACKNOWLEDGEMENT: We take this opportunity to thank all the people who have supported and guided us during the completion of this work
CONFLICTS OF INTEREST: Nil
SOURCE OF FUNDING: Nil
- Values are expressed as mean ± S.E.M. (n=6),
- #, normal control group compare with ccl4 treated group
- ### = p< 0.001,**=p< 0.01,*=p< 0.05
- *, compare with ccl4 treated group
- ***= p< 0.001, ## = p<0.01, # = p< 0.05
References:
-
Subhose V, Srinivas P, Narayana A. Basic principles of pharmaceutical science in Ayurv?da. Bull Indian Inst History Med 2005;35(2):83–92.
-
Jain JB, Kumane SC, Bhattacharya S. Medicinal Flora of Madhya Pradesh and Chhatisgarh-A review. Ind J Traditional Know 2006;5(2):237-242.
-
Muniappan R, Marutani M. Ecology and distribution of C. odorata in Asia and Pacific. In the Proceedings of the First International Workshop on Biological Control of C. odorata held from Feb 29-Mar 4; 1988, Bangkok, Thailand.
-
Sajise PE, Palis RK, Norcio NV, Lales JS. The biology of C. odorata L. King and Robinson. 1. Flowering behaviour, the pattern of growth and nitrate metabolism. Phil Weed Sci Bull 1974;1:17-24.
-
Hajra PK, Rao RR, Singh DK, Uniyal BP. Flora of India, Botanical Survey of India, Calcutta, India, 1995.
-
Howard RA. Flora of the Lesser Antilles, Leeward and Windward Islands, Harvard University, Jamaica Plain, Arnold Arboretum, USA, 1989.
-
Morton JF. Atlas of Medicinal Plants of Middle America, vol. 2, Charles C. Thomas, Springfield, Ill, USA, 1981.
-
Kokate CK. In: Practical Pharmacognosy, Preliminary Phytochemical Screening, first ed., Vallabh Prakashan, New Delhi, 1986: 111.
-
Harborne JB. Phytochemical methods: A guide to the modern technique of plant analysis (3rd edition). Chapman and Hall co. New York.1998: 1–302
-
Ecobichon DJ. The basis of toxicology testing 2nd edn, Newyork, CRC Press, pp 1999:43-46.
-
Pandi S. Sur TK, Jana Udebnath P K. Sen S& Bhattacharya D, Prevention of carbon tetra chloride-induced hepatotoxicity in rats by Adhatoda vasica leaves, Indian J Pharmol 2004;36(313):127-129.
-
Clwson GA. Mechanism of carbon tetrachloride hepatotoxicity, Pathol Immunol Res 1989;104(8):56-58.
-
Zimmerman HJ. Antituberculosis agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999; 611-21.
-
Verma S, Kaplowitz N. Hepatotoxicity of antituberculosis drugs. In, Kaplowitz N, De Leve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013; 483-504.
-
Dunnet CW. New tables for multiple comparisions with a control. Biometrics 1964; 20:482-491.
-
Gravel E, Albano E, Dianzani MU, Poli G, Slater TF, Effects of carbon tetrachloride on isolated rat hepatocytes: Inhibition of protein and lipoprotein secretion. Biochem J 1979; 178:509-512
-
Karan M, Vasisht K, Handa SS. The antihepatotoxic activity of Swertia chirata on
carbon tetrachlorideinducedhepatotoxicity in rats. Phyt Res 1999;13:24-30.
-
Van Hest R, Baars H, Kik S. Hepatotoxicity of rifampin?pyrazinamide and isoniazid preventive therapy and tuberculosis treatment. Clin Infect Dis 2004;39:488-496.
-
Fernandez?Villar A, Sopena B, Fernandez?Villar J. The influence of risk factors on the severity of anti?tuberculosis drug?induced hepatotoxicity. Int J Tuberc Lung Dis 2004;8:1499-1505.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License