IJCRR - 13(2), January, 2021
Pages: 100-105
Date of Publication: 16-Jan-2021
Print Article
Download XML Download PDF
Hypoglycemic Effects of Apigenin from Morus Indica in Streptozotocin Induced Diabetic Rats
Author: Satish Anandan, Asna Urooj
Category: Healthcare
Abstract:Introduction: Morus Indica L occupies an important position in the holistic system of Indian medicine 'Ayurveda' which has its roots in antiquity and has been followed for centuries. Objective: Methods: The hypoglycemic potential of apigenin isolated from MI leaves in streptozotocin (STZ) induced diabetic rats was studied. The rats were divided into four groups (n=6 animals in each group) viz. Group I- Normal healthy control rats (NC); Group II- STZ-induced diabetic control (DC) rats without treatment; Group III- STZ-induced diabetic rats treated with Aminoguanidine (AG) (30 mg kg-1 body weight by intraperitoneal); Group IV- STZ-induced diabetic rats treated with Apigenin (API) (50 mg kg-1 body weight was given by orally). The protective effect of API was evaluated by determining the biochemical parameters (lipid profile, liver, and kidney) and by studying the histopathological alterations in liver and kidney. Results: Diabetic control group had altered biochemical values (lipid profile, liver and kidney) when compared with the NC group. However, treatment with API showed significant improvement in the biochemical parameters and values are comparable to the NC group. Histopathological data revealed destruction of the kidney and liver architecture in DC, which was reverted in the group treated with API. Conclusions: The present findings suggest that API might be useful in the management of diabetes mellitus.
Keywords: Hyperglycemic, Mulberry, Flavonoids, Streptozotocin, Oxidative stress
Full Text:
INTRODUCTION
Diabetes mellitus (DM) is a metabolic disorder characterized by insulin resistance and pancreatic β-cell dysfunction as a consequence of unsettled hyperglycemia.1 Prolonged hyperglycaemia may lead to a variety of secondary complications such as retinopathy, nephropathy, neuropathy and cardiovascular disease.2 Type 2 diabetes mellitus (T2DM) is one of the most common forms of diabetes in worldwide. In India, around 69.2 million people are prone to T2DM and it has the second-highest number of people living with DM worldwide following China.3
Even though powerful anti-diabetic drugs are offered for the management of diabetes, there has been no drug developed which has no side effect(s) with low cost-effective.4 Therefore, natural products have stimulated a new wave of research to look for more ef?cacious agents with lesser side effects.5, 6 Traditional knowledge with its holistic and systematic approach supported by scientific documentation can serve as a novel, affordable medicine with minimum side effects.7,8
Morus Indica L. is used as traditional medicine for its hypoglycemic and diuretic properties.8In our laboratory, Morus Indica (MI) has been screened for various biological properties such as antioxidant, toxicological studies, anti-hypercholesterolemic, and anti-diabetic effect in in-vitro, ex-vivo and in-vivo models.9-12 In our previous study, Morus Indica-G4 (MI-G4) variety exhibited the highest AGEs inhibition in BSA-glucose model which could be due to the presence of polyphenols and phenolic compounds.13 Further, we isolated the bioactive compound of apigenin (API) and showed potential AGEs inhibition in all stages of protein glycation.14 Besides, API is also proved to inhibit Aldose reductase (ALR) activity, one of the major complications of diabetes (cataract) in the lens.15 Literature reports research studies carried out in the crude extract of MI and very limited studies have investigated the role of active ingredients from MI of G4 variety for their bene?cial effects on DM. Therefore, the present study was undertaken to assess the role of isolated API from MI for anti-diabetic efficacy in STZ-induced diabetic rats.
MATERIALS AND METHODS
Chemicals and reagents
Clinical diagnostics kits were purchased from M/s. Agappe Diagnostics Ltd, Kerala, India. All the chemical reagents used in this experiment were of analytical grade.
Collection of Plant material
Morus Indica G4 leaves ((ISGR Reg. No.: 050564) were collected from Centre for Sericulture Research and Technical Institute (CSRTI), Mysore district of Karnataka, India. Apigenin was isolated from 80% methanol extract of leaves by preparative HPLC and characterized through Ultra performance liquid chromatography-mass spectra (UP-LCMS), Nuclear magnetic resonance (NMR), Fourier transform infrared spectroscopy (FTIR) and Scanning electron microscope (SEM).14
Experimental rats
In this experimental study, twenty-four healthy adult male Wistar Strain rats (140-190 g), were procured from the animal house facility of the University of Mysore (UoM), Mysuru. The obtained animals were kept in polyacrylic cages. The animals were maintained under standard conditions, with a temperature of 22 ± 2?C, a regular 12/12 hour light-dark cycle. The animals were fed with pellet diet and water ad libitum. The animal experimental protocol was approved by the Ethics Committee of the UoM, Manasagangotri, Mysuru. (Animal Sanction Order No: UOM/IAEC/05/2017).
Induction of diabetes and treatment
Diabetes was induced by a single intraperitoneal injection of streptozotocin (STZ) to animals fasted overnight at a dose of 45 mg kg-1 body weight citrate buffer (pH 4.5) and the normal control rats received freshly prepared citrate buffer (pH 4.5) alone.16 Diabetic condition in rats was confirmed by measuring the altered fasting blood glucose level (by Glucometer) after 72 h of STZ injection. The rats with fasting blood glucose above 250 mg dL-1 were considered to be hyperglycemic and used for the experiment. Based on their weights using randomized block design, the rats were divided into four groups (consisting of 6 rats in each group) viz. Group I- Normal healthy control rat (NC), Group II- STZ-induced hyperglycemic rats without treatment (DC), Group III- STZ-induced hyperglycemic rats treated with Aminoguanidine (30 mg kg-1 body weight by intraperitoneal) as a positive control, Group IV- STZ-induced hyperglycemic rats treated with Apigenin (API) (50 mg kg-1 body weight was given by orally) and treated accordingly for 45 days. At the end of the study, the rats were made to fast for 12 h before blood collection. For ease of handling, before blood sampling, the rats were anaesthetized with diethyl ether. The blood samples were collected by cardiac puncture using 25 G, 1" needle. Approximately 5 ml of blood was taken and dispensed into labelled plain tubes. The blood samples were then centrifuged at 3000 rpm at 4 °C for 15 min to separate the serum. The serum was stored at -40°C until biochemical assays were carried out.
Estimation of body weight
During the experimental period, the body weights of the experimental rats were recorded on alternate weeks, i.e on day 0, 14, 28 and 45 by using a digital balance. These weights were determined at the fixed time in the morning session throughout the experimental period.
Estimation of blood glucose
Blood glucose levels of experimental rats were measured using a glucometer (Roche, Germany) by taking 0.5 µL blood from lateral tail vain onto the test strip on days of bodyweight determination.
Biochemical studies
Blood serum was used for the evaluation of lipid profile [total cholesterol (TC), triglyceride (TG) and high-density lipoprotein (HDL)], renal function test [Creatinine (CR), bilirubin (BIL), blood urea nitrogen (BUN)] and liver function test [alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) including, total protein (TP), albumin (ALB)], using commercial kits from (Agappe Diagnostics Ltd., India), according to the manufacturer’s protocol.
Histopathological studies
Small portions of the kidney and liver were fixed in 10% formaldehyde and were dehydrated with graded ethanol series (50-100%) and were embedded in paraffin. The paraffin blocks were subsequently cut into (4-5 ????m) and stained with haematoxylin and eosin (HE) dyes. The slides were examined under a microscope for histopathological changes.
Statistical analysis
The values are expressed as mean ± SD. The data were subjected to one-way ANOVA followed by Tukey's multiple comparisons test for significant difference (≤0.05) using SPSS16.0 software.
RESULTS
Changes in blood glucose level and body weight of experimental rats
The blood glucose level and body weight of all the groups of experimental rats are shown in Table 1. There was a significant elevation in the blood glucose level and a significant decrease in the body weight in diabetic control compared with normal rats. The daily administration of API for 45 days tended to decrease the blood glucose level, while the mean body weight was comparable with that of the control group.
Serum lipid profiles, renal and liver function test of experimental rats
The serum level of lipid profile [total cholesterol (TC), triglyceride (TG) and high-density lipoprotein (HDL)], renal function test [Creatinine (CR), bilirubin (BIL), blood urea nitrogen (BUN)] and liver function test [alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) including, albumin (ALB)] and total protein (TP) activities in all groups are shown in Table 2. The serum TC, TG, CR, BIL, BUN, ALT, AST and ALP levels were significantly increased in diabetic control when compared to normal control. Signi?cant (P<0.05) reduction in TC, TG, CR, BIL, BUN, ALT, AST and ALP levels along with the signi?cant (P<0.05) elevation in the HDL, ALB and TP levels were observed in API treated (50 mg Kg-1) diabetic rats when compared with the diabetic control. Improvement in the lipid pro?le, renal and liver function test, was also observed in AG (30 mg kg-1)
Histopathological changes in experimental rats
The HE stained sections of the kidney and liver of normal control, diabetic control and treatment of AG (30 mg Kg-1) and API (50 mg Kg-1) in diabetic rats are shown in Figure 1. Diabetic rats showed glomerular basement membrane thickening, mesangial expansion in the kidney, whereas in liver extensive vacuolization with the disappearance of nuclei, disaggregation of trabeculae was observed. However, 6 weeks of treatment with API in diabetic rats attenuated the destruction of tissues and showed regeneration of tissues/cells in kidney and liver architecture.
DISCUSSION
Experimentally induced diabetes through the intra-peritoneal injection of β-cytotoxic drugs such as STZ is well characterized and this chemical compound is the first choice for diabetes induction in animals.17 STZ diabetes is caused by the specific necrosis of the pancreatic β-cells results in insulin insufficiency and causes hyperglycemia. Administration of STZ rapidly produces the characteristic signs which are similar to diabetes mellitus non-ketosis hyperglycemia, The symptoms include such as increased intake of both water and food, dyslipidemia, loss of protein mass, failure to gain weight, increased blood glucose concentrations, damage to the liver and other organs resulting in major disturbance of central metabolic balance.18
Insulin plays a major role in glucose uptake into muscle and adipose tissue, deficiency of insulin leads to failure of glucose uptake resulting in a decline of stored energy.19 In our present study, API treatment to the diabetic rats resulted in a decrease in fasting blood glucose levels, which might be by stimulation of pancreatic β-cells resulting in the secretion of insulin. The observations from this study are in agreement with a study by Salem and El-Azab who showed that treatment with Aloe vera extract had blood glucose-lowering effects with a possible protective role in pancreatic β-cells of STZ-induced diabetic rats.20
In the present study, a severe decrease in body weight was observed in diabetic control rats. However, API treatment decreased the percentage of loss of the body weight in the diabetic rats which may be explained by regenerating of β- cell capacity which leads to the increases in insulin secretion21 thus con?rming the anti-hyperglycemic activity of the extract as reported by our earlier established studies.11 Insulin being an anabolic hormone promotes lipogenesis and inhibits protein catabolism promoting weight gain or maintaining the weight preventing further loss.19 These observations are in agreement with a study reporting the regeneration of β-cell and decreased weight loss following administration of Quercetin from Azadirachta indica in STZ induced diabetic rats.22 Under normal circumstances, insulin activates the enzyme lipoprotein lipase, which plays an essential role in triglyceride metabolism. However, in the diabetic state, lipoprotein lipase is not activated due to insulin deficiency, resulting in hypertriglyceridemia and hypercholesterolemia and also due to metabolic defects.23 In our study, we noticed a significant increase in serum TC and TG with a marked decrease in serum HDL levels in STZ-induced diabetic control rats. This may be due to an increase in the mobilization of free fatty acids from peripheral fat depots since insulin inhibits the hormone-sensitive lipase. Remarkably, the results of API treated groups were improved indicating the positive effect on overall lipid metabolism. Treatment with mulberry leaves has shown blood-glucose-lowering effect accompanied by amelioration in lipid abnormalities associated with diabetes in STZ induced rats.11,24
The diabetic hyperglycemia induces the elevation of renal function markers like CR, BUN and uric acid which are associated with interstitial atrophy, epithelial necrosis as well as atrophic changes in the glomeruli.25 In the present study, serum CR and BUN levels were increased in STZ-induced diabetic control rats, which may be the result of hyperglycemia, oxidation of glycated biomolecules leads to non-enzymatic reactions and polyol metabolic pathway are greatly influenced resulting in depletion of the reduced glutathione (GSH) content in the tissue and leads to increased production of ROS causing multilevel organ damage .26However, the administration of API retrieved these parameters close to normal levels. The main effect of the API may mediate their effect by directly quenching ROS induced by STZ exposure or by chelating the catalytic metal ions which are accountable for initiating peroxidation reaction, thus further confirming the antiglycation activity of the API as reported by our earlier study.27
It was observed that the liver damages in STZ-induced diabetic rats resulted in abnormal liver enzymes levels (ALP, AST and ALT). However, increased levels of these enzymes may result in low levels of serum ALB and TP was also observed in STZ induced diabetic control rats. A decrease in serum ALB and TP levels may contribute to the inhibition of oxidative phosphorylation process, leading to a reduction in protein absorption, a decline in protein synthesis, and an increase in the catabolic process.28 In the present study, a significant decrease in serum ALB and TP levels and an increase in the markers of liver injury (ALP, AST and ALT) reflected the hepatocytes damage in STZ-induced diabetic rats. This finding is consistent with the reported results.29 However, the treatment with API reverted the serum biomarkers level of liver damage (ALP, AST and ALT), ALB and TP levels near normal range which might be due to the stimulation of insulin and reduce the catabolism of protein in STZ-induced diabetic rats. These results are in line with Patel et al., which reported that anti-diabetic medicinal plants having insulin mimetic property.30 Hyperglycemia leads to increased production of ROS which is involved in the aetiology of several diabetic complications. The ROS diminish the antioxidant defences of the cell thus making it more susceptible to oxidative damage. It further targets lipid, carbohydrate and nucleic acid leading to their oxidation which further leads to dysfunction of organ architecture and its function.26 In our study, diabetic control rats showed glomerular basement membrane thickening, mesangial expansion and extensive vacuolization with the disappearance of nuclei, disaggregation of trabeculae of kidney and liver respectively. Treatment with API signi?cantly reduced the aforementioned alterations and thereby protecting the renal and hepatocyte, thus confirming the antioxidant activity of the extract as reported by our earlier study.9 These observations are in agreement which was reported that Mucuna pruriens seeds are capable of exerting positive structural changes in pancreas and liver through its antioxidant and anti-diabetic properties.31
CONCLUSION
This study shows the ameliorative e?ect of apigenin from Morus Indica in STZ induced diabetic rats that can be bene?cial to maintain the glycemic level in diabetes and may prevent the secondary complications of hyperglycemia. Its hypoglycemic activity seems to be principally by means stimulations of insulin secretion and which may contribute to the normalization of biochemical parameters of lipid profile, renal and liver function test. Based on the outcomes of the present study, it is proven that apigenin from Morus Indica has potential hypoglycemic activities and the result scientifically valid their use in traditional medicine. However, further studies are required to understand the mechanisms of action underlying the beneficial effects of Apigenin on this pathology
ACKNOWLEDGEMENTS
The first author is also thankful to my colleagues for assisting in animal experiments. We are also grateful to the Institution of Animal Health and Veterinary Biologicals, Mysuru and Institution of Excellence (IOE) University of Mysore, Mysuru for providing instrumentation facilities.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
SOURCE OF FUNDING:
This work was financially supported by ICMR, New Delhi and DRS-UGC-SAP-II, New Delhi.
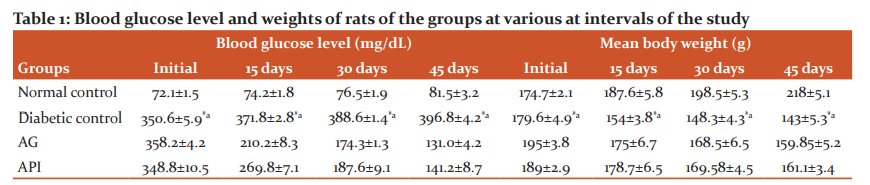
Initial refers to 1st day of the treatment after diabetic induction and final values were taken before sacrifice. Values are the mean of values for six rats with standard deviation. AG: Aminoguanidine (30 mg Kg-1) treated group, API:Apigenin (50 mg Kg-1) treated group. Values are mean of each group (n=6) and ± indicate standard errors according to Tukey’s Multiple Comparison Test, *p < 0.05 (Normal vs Diabetic), ap < 0.05 (Diabetic vs AG and API). AG: Aminoguanidine (30 mg Kg-1) treated group, API:Apigenin (50 mg Kg-1) treated group.
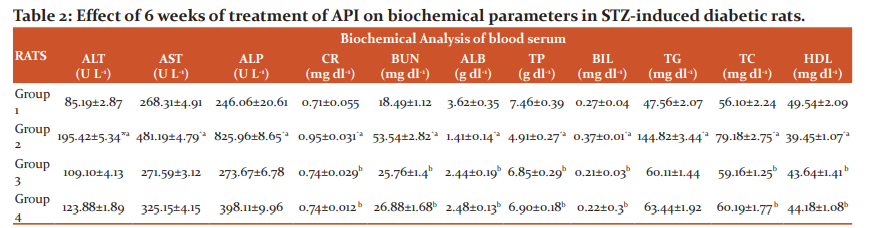
Note: Lipid profile [total cholesterol (TC), triglyceride (TG) and high-density lipoprotein (HDL)], Renal function test [Creatinine (CR), bilirubin (BIL), blood urea nitrogen (BUN)] and Liver function test [alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) including, total protein (TP), albumin (ALB)], Values are mean of each group (n=6) and ± indicate standard errors according to Tukey’s Multiple Comparison Test, *p < 0.05 (Normal vs Diabetic), ap < 0.05 (Diabetic vs AG and API) and bp > 0.05 (AG vs API). AG: Aminoguanidine (30 mg Kg-1) treated group, API:Apigenin (50 mg Kg-1) treated group. Group 1- Healthy control rats; Group 2- STZ-induced hyperglycemic rats without treatment; Group 3- STZ-induced hyperglycemic rats treated with AG; Group 4- STZ-induced hyperglycemic rats treated with API.
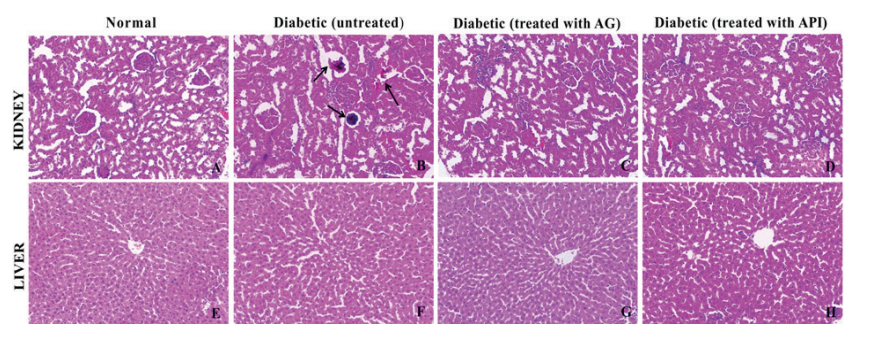
Figure 1: The histopathological effect of API in STZ-induced diabetic rats was carried in the kidney and liver. (A) and (E) are the cross sections of Kidney and Liver respectively of Normal rats. (B) and (F)are the cross sections of Kidney and Liver respectively of STZ induced diabetic control rats, (C) and (G)are the cross sections of Kidney and Liver respectively of STZ induced diabetic rats treated with aminoguanidine , (D) and (H)are the cross sections of Kidney and Liver respectively of STZ induced diabetic rats treated with API. AG: Aminoguanidine (30 mg Kg-1) API:Apigenin (50 mg Kg-1).
References:
-
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014 Jan 1;37(1): S81-90.
-
Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes 2008 Apr 1;26 (2):77-82.
-
Sen A. Health: perception versus observation: self-reported morbidity has severe limitations and can be extremely misleading. BMJ 2002;324(7342): 860–861.
-
Chaudhury A, Dufour C, Dendi R, Sena V, Kraleti S, Chada A, et al. Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Front Endocrinol 2017;8:6.
-
Rey-Ladino J, Ross AG, Cripps AW, McManus DP, Quinn R. Natural products and the search for novel vaccine adjuvants. Vaccine 2011;29(38):6464-6471.
-
Katiyar C, Gupta A, Kanjilal S, Katiyar S. Drug discovery from plant sources: An integrated approach. Ayurveda 2012;33(1):10-9.
-
Patwardhan B, Vaidya AD, Chorghade M. Ayurveda and natural products drug discovery. Curr Sci 2004 Mar 25:789-799.
-
da Silva Almeida JR, Souza GR, da Cruz Araújo EC, Silva FS, de Lima JT, de Araújo Ribeiro LA, et al. Medicinal plants and natural compounds from the genus Morus (Moraceae) with hypoglycemic activity: a review. Glucose Toler 2012:189.
-
Arabshahi-Delouee S, Urooj A. Antioxidant properties of various solvent extracts of mulberry (Morus Indica L.) leaves. Food Chem 2007;102(4):1233-1240.
-
Reddy PV, Urooj A. Inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase (ex vivo) by Morus Indica (Mulberry). Chinese J Biol 2014; 2014.
-
Devi DV, Urooj A. Antihyperglycemic and hypolipidemic effect of Morus Indica L. in streptozotocin-induced diabetic rats. Ann Phytomed 2014;3(2):55-59.
-
Reddy PV, Sreenivas N, Urooj A. Acute toxicological studies of leaf extracts of Morus Indica L. in rats. Ann Phytomed 2016;5(2):108-102.
-
Anandan S, Kotebagilu NP, Shivanna LM, Urooj A. Inhibitory potency of C-glycosyl flavonoids from morus sp. on advanced glycation end products. J Biol Active Prod 2017;7(5):391-400.
-
Anandan S, Urooj A. Bioactive Compounds from Morus Indica as Inhibitors of Advanced Glycation End Products. Ind J Pharm Sci 2019;81(2):282-292.
-
Anandan S, Mahadevamurthy M, Urooj A. Ex vivo and in silico Molecular Docking Studies of Aldose Reductase Inhibitory Activity of Apigenin from Morus Indica L. J Young Pharm 2019;11(1):483-486.
-
Furman BL. Streptozotocin?induced diabetic models in mice and rats. Curr Protoc Pharmacol 2015;70(1): 5.47.1-5.47.20.
-
Ward DT, Yau SK, Mee AP, Mawer EB, Miller CA, Garland HO, Riccardi D. Functional, molecular, and biochemical characterization of streptozotocin-induced diabetes. J Am Soc Nephrol 2001;12(4):779-790.
-
Wei M, Ong L, Smith MT, Ross FB, Schmid K, Hoey AJ, et al. The streptozotocin-diabetic rat as a model of the chronic complications of human diabetes. Heart Lung Circ 2003;12(1):44-50.
-
Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005 May;26(2):19-39.
-
Salem MY, El-Azab NE. The possible protective role of aloe vera extracts in pancreatic β cells of experimentally induced diabetic rats: a histological and immune histochemical study. Egyptian J Histol 2014 Sep 1;37(3):571-578.
-
Fernstrom MH, Fernstrom JD. Large changes in serum free tryptophan levels do not alter brain tryptophan levels: studies in streptozotocin-diabetic rats. Life Sci 1993;52(11):907-916.
-
Aguirre L, Arias N, Teresa Macarulla M, Gracia A, P Portillo M. Beneficial effects of quercetin on obesity and diabetes. Open Nutraceu J 2011;4(1):189-198.
-
Shah SS, Shah GB, Singh SD, Gohil PV, Chauhan K, Shah KA, Chorawala M. Effect of piperine in the regulation of obesity-induced dyslipidemia in high-fat diet rats. Ind J Pharmacol 2011;43(3):296-299.
-
Andallu B, Kumar AV, Varadacharyulu NC. Lipid abnormalities in streptozotocin-diabetes: amelioration by Morus indica L. cv Suguna leaves. Int J Diabetes Dev 2009 Jul;29(3):123-128.
-
Jain D, Saha S. Antioxidant and antihyperglycaemic effects of naringenin arrest the progression of diabetic nephropathy in diabetic rats. Egypt pharmaceut J 2017;16 (3):144-151.
-
Niedowicz DM, Daleke DL. The role of oxidative stress in diabetic complications. Cell Biochem Biophys 2005;43(2):289-330.
-
Anandan S, Siddiqi S, Siraj SF, Asna U. Protective Effect of Apigenin from Morus indica. L against Methylglyoxal Induced Oxidative DNA Damage. Int J Pharm Biol Sci. 2019.9(20):173-178.
-
Ghanbari E, Nejati V, Khazaei M. Improvement in serum biochemical alterations and oxidative stress of liver and pancreas following use of royal jelly in streptozotocin-induced diabetic rats. Cell J 2016;18(3):362-370.
-
Nithiya T, Udayakumar R. Hepato and renal protective effect of phloretin on streptozotocin-induced diabetic rats. J Biomed Pharm Sci 2018;1(105):3-6.
-
Patel DK, Prasad SK, Kumar R, Hemalatha S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac J Trop Biomed 2012;2(4):320-330.
-
Rajesh R, Arunchandra SS, Anandraj KV, Manimekalai K, Dhananjay K, Rajasekar. The effect of Mucuna pruriens seed extract on pancreas and liver of diabetic Swistar rats. Int J Curr Res Rev 2016;8(4):61-67.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License