IJCRR - 13(2), January, 2021
Pages: 90-94
Date of Publication: 16-Jan-2021
Print Article
Download XML Download PDF
Lipid Peroxidation and Hepatoprotective Activity of Bauhinia Vahli Against Carbon Tetra Chloride Induced Toxicity
Author: Amit Kumar Nigam, Phool Chandra, Zeashan Hussain, Neetu Sachan
Category: Healthcare
Abstract:Introduction: Liver cirrhosis has become a serious health problem because of the wider use of prescribed medications with adverse reactions in the modern life of today or drug misuse. Objective: In the present study, the hepatoprotective activity of the aerial part of Bauhinia vahlii was investigated against the carbon tetrachloride-induced hepatotoxicity model in Wistar albino mice. Methods: Glutathione (GSH), Glutathione Peroxidase (GPx), Glutathione S-transferase (GST), Superoxide dismutase (SOD), Multiple Organ Dysfunction Syndrom (MOD) were measured in liver and blood samples. The acute toxicity study of the selected plant showed that there is no mortality or adverse reaction at the fixed dose of 2000 mg/kg. The methanolic extract at 200 and 400 mg/kg body weight respectively were administered daily to study the hepatoprotective effect in carbon tetrachloride-induced hepatotoxic model for 7 days. Results: A remarkable decrease was observed in bilirubin, alkaline phosphatase (ALP), serum glutamic-oxaloacetic transaminase (SGOT), and serum glutamic-pyruvic transaminase (SGPT) levels in the treatment group as compared to the hepatotoxic group. In a histopathological study, hepatocytic necrosis and inflammation in the centrilobular region with portal triaditis was found in hepatotoxicity induced group whereas minimal inflammation with moderate portal triaditis and normal lobular architecture observed in extract treated groups. Conclusion: From the observation, it can be concluded that the methanolic extract of Bauhinia vahlii has the potential to revert the hepatic injury induced by carbon tetrachloride. Comparatively, the methanolic extract at 400 mg/kg w/w resulted in a significant hepatoprotective effect. The standard drug silymarin was used for comparison.
Keywords: Hepatoprotective activity, Lipid peroxidation, Bauhinia vahlii, Silymarin, Carbon tetrachloride
Full Text:
INTRODUCTION
The liver is the largest organ in the body weighing 1400-1600 gm. in males and 1200-1400 gm. in females. There are two main anatomical lobes right lobe and left lobe, the right is being about six times the size of the left lobe. It produces bile, an alkaline compound which aids in digestion, via the emulsification of lipids. Besides, it aids the metabolism of carbohydrate, protein and fat, detoxification, secretion of bile, and storage of vitamins.1,2 The primary function of this organ in the removal of substances from the portal circulation makes it susceptible to first and persistent attacks by offending foreign compounds, culminating in liver dysfunction.3
Liver cirrhosis has become a serious health problem because of the wider use of prescribed medications with adverse reactions in the modern life of today or drug misuse. The current research has targeted on finding new therapeutic alternatives and analyzing their mechanism to get rid of the signalling routes and reduce the loss induced on the liver.4 The numbers of compounds of natural origin are generally used as possible health care options and they are being experimented on various animal models.5,6 Many medicinal plants with hepatoprotective activity have been reported by several researchers.7,8 In continuation of the search of hepatoprotective agents, Malu Creeper was undertaken to investigate the active constituents and its medicinal value. Bauhunia vahlii (Malu Creeper) is a tree of large family Leguminosaceae (subfamily- Caesalpiniaceae); it is traditionally used to treat the liver cirrhosis.9
Bauhinia genus of family Caesalpiniaceae consists of about 15 species that occur in India. Some of them are shrubs or trees, while a few are climbers.10 Bauhinia vahlii is a giant climber shrub and one of the most common Indian Bauhinia species. The species is distributed in the Sub-Himalayan region and also found in Assam, Central India, Bihar, Eastern, and the Western Ghats.11 Bauhinia vahlii Wight and Arn belonging to family Caesalpiniaceae is a giant evergreen shrub having white flowers. Various part of Bauhinia vahlii is used, as the seeds possess tonic and aphrodisiac properties and leaves are demulcent and mucilaginous. The plant is reported to possess antibacterial,12 anti-inflammatory, anti-diabetic13,14,15 antioxidant16 and anti-hyperlipidemic activity.17 It is also used for the treatment of dysentery, diarrhoea, haemorrhoids, piles, oedema, laxative, anthelmintic, astringent, antileprotic, wound healing, antigoitrogenic, antitumor, the antidote for snake poisoning, dyspepsia, carminative.18
Bauhinia vahlii is the largest creeper in India and is called adattige in Telugu and asamantaka in Sanskrit. It has been reported to contain amino acids, proteins,19 minerals,20 and flavonoids.21 They have various medicinal activity, Dried pods, without seeds, yielded 4 new constituents, viz. methyl 4-O-methyl gallate (0.08%), methyl gallate (0.26%), (+)-mopanol (0.07%) and (+)-catechin (0.11%).22 It has also contained betulinic acid, triterpene, campesterol, and steroid. Even though the plant was traditionally used to treat liver disease, but there is no such documented evidence of hepatoprotective activity. Because of this, it is of considerable interest to investigate the above-mentioned plant with the hope to obtain a safe and potent hepatoprotective agent.
MATERIALS AND METHODS
Collection and identification of plant sample
The fresh aerial parts of Bauhinia vahlii were collected from surrounding of Chandi Devi Temple located in Haridwar, Uttarakhand, India. The selected plant was authenticated by CSIR Laboratory, National Botanical Research Institute, Lucknow (CSIR-NBRI). The authentication reference number is NBRI/CIF/530/2018.
Preparation of Plant Extract
The aerial parts were washed with running tap water, air-dried under the shade at room temperature, and then ground to a coarse powder and stored in an airtight container. The size of the dried parts was reduced, powdered and about 250 g of powder was continuously extracted with methanol and water by soxhlet extractor at room temperature for three days. The methanolic extract was filtered and concentrated to a dark viscous mass (Yield 21.7 % w/w) under reduced pressure at 45-50°C. The dried extract was suspended in distilled water and was further fractionated by using different polarity-based solvents such as n-hexane, ethyl acetate; the obtained dark brown to green colour crude extract was stored in an airtight container at 6-10°C for further studies.23
Animals
Albino mice of weight ranges from 120-150 gm of female mice were used for the study. Female Mice were kept in the animal house of the pharmacy department at maintained room temperature of 22-25°C. During the whole experimental study animals kept in light and dark conditions. The experiments were carried according to guidelines of the Institutional Animal Ethics Committee approved the experimental protocol and animals were maintained under standard conditions in an animal house. Ref. no: 2014/PO/Re/S/18/CPCSEA.
Acute toxicity study
The acute toxicity study was done according to the Organization for Economic Cooperation and Development guidelines (OECD 423). In acute toxicity study, methanolic extract and aqueous extract were determined on Albino mice of weight ranges from 25-30 gm of female mice. Acute toxicity was calculated as per OECD 423 guidelines and LD50 of test compounds were found at 2000 mg/kg.w/w.
Carbon tetrachloride-induced liver toxicity
Animals were divided into five groups (each group contains 6 animals). Group, I served as vehicle control, which received liquid paraffin. Groups II-V were treated with CCl4 in liquid paraffin (1:2) at the dose of 1 ml/kg body weight intraperitoneal once in every 72 h for 14 days. Groups IV and V were treated with Bauhinia vahlii Methanolic extract (BBME) at the doses of 200 and 400 mg/kg body weight, respectively. Group III was administered with standard drug silymarin at a dose of 25 mg/kg body weight orally. BBME treatment was started 10 days before Carbon tetrachloride administration and continued until the end of the experiment.24
Evaluation of liver damage
At the end of the experiment, after 72 hrs toxicity of CCl4, blood samples were collected from retro-orbital plexus from the overnight fasted animals, after anaesthetized with 100 mg/kg ketamine, i.p. The blood samples were taken with 20 μl Ethylene diamine tetraacetic acid (EDTA) (5%) in each Eppendorf and centrifuged at 5000 rpm for 20 min (Sigma 3K30, UK). The supernatant (serum) was separated with the help of a micropipette and placed it in a new Eppendorf with well labelled and stored at -80°c for further analysis. The homogenate was centrifuged at 4°C for 5 min at 3000 r/min and the supernatant used for the estimation of viscous oxidative stress markers. (Biochemical and antioxidant estimation). The opposite liver tissue specimens were used for histopathological examination.25,26 Biochemical parameters like Serum glutamate oxaloacetate transferase (SGOT), Serum glutamate pyruvate transferase (SGPT), alkaline phosphatase (ALP) and Serum bilirubin were estimated by reported methods.27,28
Statistical analysis
The results of the study were expressed as mean±SEM (Standard error of the mean). The student t-test and analysis of variance (ANOVA) were used followed by Newman-Keul’s multiple comparison tests to analyze the experimental data for its significance.
RESULTS
Histopathological Investigation
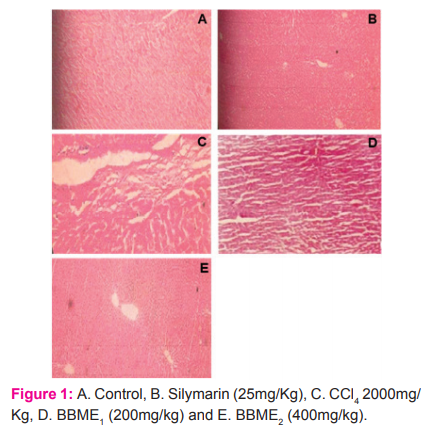
Control (Group I): Hepatocytes of the normal control group showed normal lobular architecture of the liver (Figure 1A).
Toxic Control Groups (II): Hepatocytic necrosis and inflammation region were observed in the liver treated with carbon tetrachloride (Figure 1B).
Silymarin 25 mg/kg (Groups III): Silymarin pretreated group showed normal hepatocytes normal architecture (Figure 1C).
Methanolic extract 200 mg/kg and 400 mg/kg (Groups IV & V): Methanolic extract 200 mg/kg and 400 mg/kg treated group showed recovery of hepatic parenchyma, mild congestion and microvesicular changes (Figure 1D & 1E).
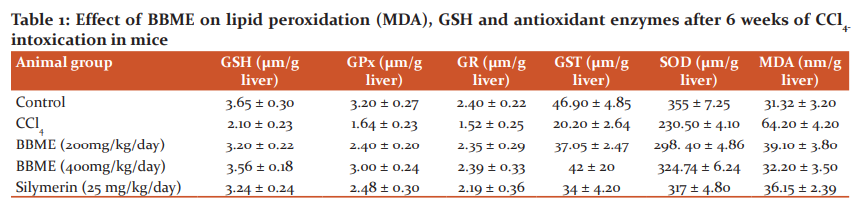
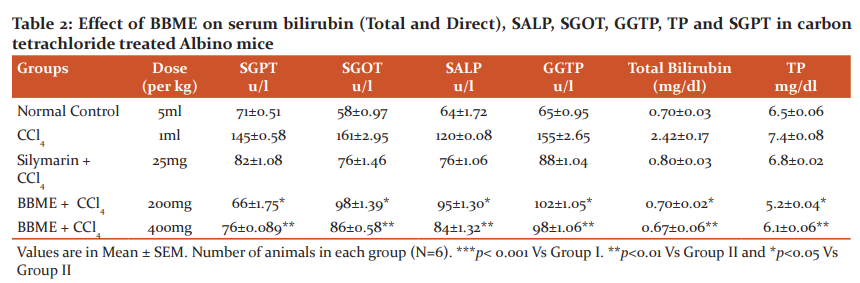
The phytochemical analysis of an extract of aerial parts of Bauhinia Vahli confirmed the presence of various phytoconstituents i.e. alkaloids, flavonoids, glycosides, terpenoids, and steroids. The extract is devoid of toxicity up to 2000 mg/kg in Wistar albino mice. In Carbon, tetrachloride treated mice, the levels of Bilirubin (Total & Direct), serum glutamic-oxaloacetic transaminase (SGOT), and serum glutamic-pyruvic transaminase (SGPT), and Alkaline phosphatase was significantly elevated. Silymarin (25 mg/kg) showed a significant decrease in Bilirubin (Total & Direct), SGOT, SGPT, and Alkaline phosphate level. Treatment with methanolic extract of Bauhinia Vahli at 200 mg/kg and 400 mg/kg showed a significant decrease in Bilirubin (Total & Direct), SGOT, SGPT, and Alkaline phosphatase level. Similarly, the treatment of aqueous extract at 200 and 400 mg/kg but 200 mg/kg showed a significant decrease in the above-mentioned biomarkers. However, 400 mg/kg w/w had shown a profound reduction of the above markers. The detailed result of the biochemical analysis is given in tables 1 & 2. The histopathological studies of the normal liver sections showed the hepatic cells with well-preserved cytoplasm, prominent nucleus, and central vein (Figure 1A). In rats treated with Carbon tetrachloride, the normal architecture of the liver was completely lost with the appearance of centrilobular necrosis, lymphocytes infiltration of the periportal area, and fatty changes were observed (Figure 1B). The mice administrated with silymarin (25 mg/kg) exhibited a significant reversal of the hepatic damage caused by Carbon tetrachloride (Figure. 1C). Methanolic extract at a dose of 200 mg/kg and 400 mg/kg had shown some improvement in damaged hepatocytes whereas the aqueous extract at 200 and 400 mg/kg had shown remarkable improvement in damaged hepatocyte which is evidenced from reversal of damaged hepatocytes to normal hepatic architectural pattern with mild hepatitis (Figure. 1D & 1E).
Discussion
CCl4 intoxication is a widely used experimental model for liver injury. The highly hepatotoxic metabolites, namely, trichloromethyl radicals (CCl3* and CCl3O2*) are generated during the metabolic activation of CCl4 by cytochrome P-450. These radicals have a central role in the initiation of lipid peroxidation, inflammation, and fatty changes of the liver.29,30 Moreover, CCl4 intoxication is associated with oxidative stress since theCCl3* and CCl3O2* radicals alter the antioxidant state of the liver by deactivating the hepatic antioxidant enzymes including SOD, GPx, GR, and GST. Trichloromethyl radicals also react with the sulfhydryl groups of GSH leading to its deactivation.31 In the present study, CCl4 treatment markedly increased the levels of AST, ALT, and ALP. The leakage of the marker enzymes into the blood was associated with marked necrosis, loss of hepatic architecture, hydropic degeneration, fatty changes, Kupffer cell hyperplasia, central vein congestion, and infiltration of the liver by lymphocytes. The MDA level in the liver tissue was markedly increased in response to CCl4 intoxication, indicating oxidative damage of the liver. CCl4 administration also reduced the levels of GPx, SOD, GST, GSH, and GR in the liver tissue compared to the normal mice. The results of the present study demonstrated that treatment with BBME returned the increased MDA to its normal level. The inhibitory effect against lipid peroxidation suggested that BBME could prevent the liver injury induced by free radicals along with the subsequent pathological changes in the liver.33
The marked reduction in the leakage of liver enzymes into the serum also confirmed the inhibitory effect of BBME against lipid peroxidation. In contrast, the GSH, GPx, SOD, GST, and GR levels were markedly improved compared to the silymarin-treated group. Modulation of these antioxidant defences contributed to the antioxidant and hepatoprotective activity of BBME. The remarkable hepatoprotective and antioxidant effect of Bauhinia vahlii Methanolic extract (BBME) may be attributed to a synergistic effect between these compounds.32, 34
Conclusion
Based on the results of this study, the hepatoprotective effect of BBME is attributed to its ability to reduce the rate of lipid peroxidation, to enhance the antioxidant defence status, and to guard against the pathological changes of the liver induced by CCl4 intoxication. The hepatoprotective activity of BBME is concluded to be partly mediated by the antioxidant effect of the extract. Advanced tools and equipment are needed for identification, isolation, and purification of the active ingredients of the hepatoprotective activity and to examine their efficacy and safety through controlled clinical trials. There should be a motivation plan to Medicinal Plants Research Centre in Sudan to create cooperative collaborative research activities among the involved institutions.
ABBREVIATIONS
ALP, Alkaline phosphatase; BBME, Bauhinia vahlii Methanolic extract, CCl4, Carbon tetrachloride; GPx, Glutathione Peroxidase; GSH, Glutathione; GST, Glutathione S-transferase; MOD, Multiple Organ Dysfunction Syndrom; SGOT, Serum Glutamic Oxaloacetic Transaminase; SGPT, Serum Glutamic Pyruvic Transaminase; SOD, Superoxide dismutase.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Animals were used for studies that are the basis of this research.
ACKNOWLEDGMENTS
Authors are thankful to CSIR-National Botanical Research Institute, Lucknow, Uttar Pradesh, India for authentication of selected Indian medicinal plant. Authors are also thankful to Er. Mahesh Goel, Chairman, Goel Group of Institutions, Lucknow, India for providing the research facilities to the compilation of present study.
CONFLICT OF INTEREST
The authors declare that they have no financial conflict of interest.
FUNDING SOURCES
Nil
References:
-
Afolayan AJ, Adewusi EA. A review of natural products with hepatoprotective activity. J Med Plant Res 2010;4: 1318-1334.
-
Ahsan MR, Islam KM, Bulbul IJ. Hepatoprotective activity of Methanol Extract of some medicinal plants against carbon tetrachloride-induced hepatotoxicity in rats. Eur J Sci Res 2009;37:302-310.
-
Banskota AH, Tezuka Y, Adnyana IK, Xiong Q, Hase K, Tran KQ, et al. Hepatoprotective effect of Combretum quadrangular and its constituents. Biol Pharm Bull 2003;23:456-460.
-
Daly AK, Donaldson PT, Bhatnagar P, Shen Y, Peer I, Floratos A. HLA-B5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet2009;41:816-819.
-
Yang H, Sung SH, Kim YC. Two new hepatoprotective stilbene glycosides from Acer mono leaves. J Nat Prod 2005;68:101-103.
-
Wang N, Li P, Wang Y, Peng W, Wu Z, Tan S. Hepatoprotective effect of Hypericum japonicum extract and its fractions. J Ethnopharmacol 2008;116:1-6.
-
Alshawsh MA, Abdulla MA, Ismail S, Amin ZA. Hepatoprotective effects of Orthosiphon stamineus extract on thioacetamide-induced liver cirrhosis in rats. Evid Based Complement Alternat Med 2011;2011:1-6.
-
Amin ZA, Bilgen M, Alshawsh MA, Ali HM, Hadi AHA, Abdulla MA. Protective role of Phyllanthus niruri extract against thioacetamide-induced liver cirrhosis in the rat model. Evid Based Complement Alternat Med 2012;2012:1-9.
-
Garudapuran, Geeta press book, 2013, path 187.
-
Krishnamurthi A, Manjunath BL, Sastri BN, Deshaprabhu SB, Chadha YR. The Wealth of India. Dictionary of Indian Raw Materials & Industrial Products, First supplement series (Raw Materials). NISCAIR New Delhi 2004;1:119-119.
-
Chauhan R, Saklani S. Bauhinia vahlii: plant to be explored. Int Res J Pharma 2013;4:5-9.
-
Pritipadma P, Sikha S, Abhishek P, Priyanka D Goutam G. Antimicrobial and Immunomodulatory Activities of Methanolic Extract of Bauhinia vahlii. Res J Pharm Biol Chem Sci 2015;6:655-660.
-
M Nirmala M, Girija K, Lakshman K, Divya T. Hepatoprotective activity of Musa paradisiaca on experimental animal models. Asian Pac J Trop Biomed 2012;2:1383-1387.
-
Elbanna AH, Nooh MM, Mahrous EA, Khaleel AE, Elalfy TS. Extract of Bauhinia vahlii Shows Antihyperglycemic Activity, Reverses Oxidative Stress, and Protects Against Liver Damage in Streptozotocin-induced Diabetic Rats. Pharmacogn Mag 2017;3:607-612.
-
Saravanan KS, Madhavan V. Antidiabetic activity of Bauhinia vahlii wt. and arn. (Caesalpiniaceae) root a botanical source for the Ayurveda drug murva. Asian J Pharm Clin Res 2019;12:359-362.
-
Singh KL, Singh DK, Singh VK. Multidimensional uses of Bauhinia variegate. Am J Phytomed Clin Therap 2016;4:58-72.
-
Rajani GP, Ashok P. In vitro antioxidant and antihyperlipidemic activities of Bauhinia variegata Linn. Int J Pharmacol 2009;41:227-232.
-
Bansal V, Malviya R, Malaviya D, Sharma PK. Phytochemical, Pharmacological Profile and Commercial Utility of Tropically Distributed Plant Bauhinia variegate. Global J Pharmacol 2014;8:196-205.
-
Rajaram N, Janardhanan K. Chemical composition and nutritional potential of tribal pulses Bauhinia purpurea, B. racemosa and B. vahlii. J Sci Food Agri 1991;55:423–46.
-
Sastri BV, Shenolikar IS. Nutritive value of two unusual foods, adda (Bauhinia vahlii) and marking nut (Semecarpus anacardium) kernels. Indian J Med Res 1974;62:1673–1677.
-
Sultana S, Ilyas M, Kamil M, Shaida WA. Chemical investigation of Bauhinia vahlii (Leguminoceae). J Indian Chem Soc 1985;62:337–378.
-
Kumar RJ, Krupadanam GLD, Srimannarayana G. Phenolic constituents from the pods of Bauhinia vahlii, Fitoterapia 1990;61:475-476.
-
Sultana S, Ilyas M, Kamil M, Shaida WA. Chemical Investigation of Bauhinia vahlii Wight And Arnott Leaves Grown In Egypt. J Indian Chem Soc 1985;62:337-338.
-
Ali SA, Sharief NH, Mohamed YS. Hepatoprotective Activity of Some Medicinal Plants in Sudan. Evid Based Complement Alternat Med 2019;2019:1-16.
-
Pritipadma P, Sikha S, Abhishek P, Priyanka D Goutam G. Antimicrobial and Immunomodulatory Activities of Methanolic Extract of Bauhinia vahlii. Res J Pharm Biol Chem Sci 2015;6:656-657.
-
Kind PR. Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J Clin Pathol 1954;7:322–326.
-
Reitman S, Frankel S.Acolorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 1957;28:56–63.
-
Asha VV, Sheeba MS, Suresh V, Wills PJ. Hepatoprotection of Phyllanthus maderaspatensis against experimentally induced liver injury in rats. Fitoterapia 2007; 78:134-135.
-
Malloy HT, Evelyn KA. The determination of bilirubin with the photoelectric colourimeter. J Biol Chem 1937;119:481-490.
-
Srivastava A, Shivanandappa T. Hepatoprotective effect of the root extract of Decalepis hamiltonii against carbon tetrachloride-induced oxidative stress in rats. Food Chem 2010;118:2010:411–417.
-
Lee SJ, Lim KT. The glycoprotein of Zanthoxylum piperitum DC has a hepatoprotective effect via an anti-oxidative character in vivo and in vitro. Toxicol In-Vitro 2008;22:376–385.
-
Han X, Shen T, Lou H. Dietary polyphenols and their biological significance. Int J Mol Sci 2007;8:950–988.
-
Al-Isawi JKT, Al-Jumaily EF. Antioxidants and Hepatoprotective Study of a Purified
Bauhinia variegate Leaves and Flowers against Carbon Tetrachloride-Induced Toxicity
in Experimental Rats. Biomed Pharmacol J 2019;12:411-422.
-
Rayese A, Vaseem R, Manik S. Hepatoprotective Activity of Ethyl Acetate Extract of Adhatoda Vasica in Swiss Albino Rats. Int J Cur Res Rev 2013;05(6):16-21.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License