IJCRR - 13(1), January, 2021
Pages: 161-164
Date of Publication: 05-Jan-2021
Print Article
Download XML Download PDF
Role of Maternal Serum Ferritin in Gestational Diabetes Mellitus
Author: Feroz Alam, Surabhi Gautam, Nasreen Noor, Shagufta Moin
Category: Healthcare
Abstract:Introduction: Gestational diabetes mellitus (GDM) is one of the most common medical disorders complicating pregnancy, and it usually resolves following delivery. However, GDM can affect the immediate maternal and perinatal outcomes, besides, it can also have long-lasting health consequences for both the mother and the newborn. Evidence from experimental studies has shown that higher than normal ferritin concentrations can lead to pancreatic \? cell dysfunction and impaired glucose metabolism and GDM.
Objective: Estimation of serum ferritin in early pregnancy in normal controls and women suffering from GDM.
Methods: 50 Pregnant women (24-28 weeks gestation) were divided into GDM cases (n=35) and normal controls (n=15). Blood samples were collected for estimation of HbA1c and serum ferritin and the results were analysed statistically.
Results: The values of initial DIPSI 2 hr blood glucose, and later HbA1c and serum ferritin were significantly higher (p< 0.05) in GDM cases in comparison to the control group.
Conclusion: Serum ferritin is significantly higher in patients of GDM, pointing towards a possible role of oxidative stress in GDM as well as fetal intra-uterine and postpartum well being.
Keywords: Iron, Gestational diabetes mellitus, Ferritin
Full Text:
INTRODUCTION
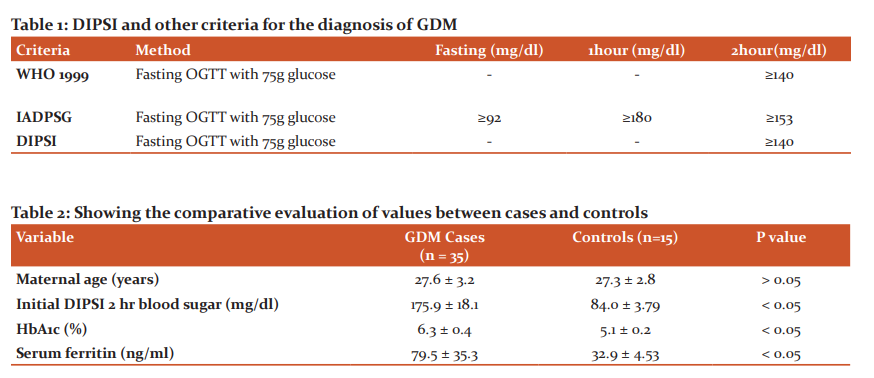
Diabetes is a global health epidemic, and India is one of the most affected countries, the prevalence of diabetes in urban India varies from 4.6%-14% and in rural India from 1.7%-13.2%. Presently, it is estimated that there are about 62 million cases of diabetes in India, and this figure will rise to 79.4 million by the year 2025. Diabetes is a major public health problem in India with prevalence rates reported to be between 4.6% and 14% in urban areas, and 1.7% and 13.2% in rural areas. India has an estimated 62 million people with Type 2 diabetes mellitus (DM), this number is expected to go up to 79.4 million by 2025.1 With this increase in diabetes prevalence, there seems to be a corresponding increase in the prevalence of Gestational Diabetes Mellitus (GDM). Gestational diabetes mellitus (GDM) is a common pregnancy complication, which in simple terms is spontaneous hyperglycemia developing during pregnancy. The American Diabetes Association (ADA) formally classifies GDM as “diabetes first diagnosed in the second or third trimester of pregnancy that is not either preexisting type 1 or types 2 diabetes”.2 GDM is said to affect approximately about 14% of pregnancies worldwide, representing approximately 18 million births annually. In India, the prevalence of gestational diabetes has been reported to range from 3.8% in Kashmir, to 6.2% in Mysore, 9.5% in Western India and 17.9% in Tamil Nadu. Also, in some recent studies using different criteria for diagnosis, prevalence rates as high as 35% from Punjab and 41% from Lucknow have been observed. These geographical differences in prevalence can be attributed to differences in age and/or socioeconomic status of pregnant women in different regions. At any given point of time, India is estimated to have 4 million pregnant females suffering from GDM.3 It is estimated that about 4 million women are affected by GDM in India, at any given time point.3 Despite, the ever-increasing risks associated with GDM, no widespread agreement exists among experts to diagnose it warranting treatment in a pregnant woman. In India, Diabetes in Pregnancy Study Group in India (DIPSI) criteria is used as the norm to diagnose GDM, especially in the community setting. The DIPSI recommends a non-fasting OGTT (Table-1) based on the belief that fasting OGTT would be logistically difficult in pregnant women in the community as it required them to return to the clinic on a separate day. The evidence basis of the DIPSI criteria is a single-centre study comparing non-fasting OGTT with World Health Organization (WHO) 1999 criteria, showing 100% sensitivity and specificity.3
Iron is stored in the body as ferritin, and measurement of ferritin in serum reflects adequately the pool of iron present in bone marrow macrophages. Ferritin is also an acute phase reactant and it is increased in many chronic diseases and inflammation. Increased cellular ferritin has been linked to insulin resistance and pancreatic β-cell dysfunction. Ferritin the major iron storage protein has a function in iron metabolism. Serum ferritin concentration displays the measure of body iron stores because it is highly correlated with bone marrow iron. High serum ferritin levels have been demonstrated in many chronic disorders and vascular inflammation. Mildly elevated body iron stores have been associated with elevations in glucose homeostasis indices. A significant correlation between higher serum ferritin levels and insulin resistance syndrome has been shown. Elevated serum ferritin levels were associated with greater than the two-fold increased risk of development of type 2 diabetes in the Finnish population. A strong association between higher serum ferritin concentration and newly diagnosed type 2 diabetes was observed among a U.S. population as well.4 Studies have suggested that raised serum ferritin levels were found in women with non-insulin-dependent diabetes as well as in women with GDM, and it was reported to be associated with glycemic control.5 It was postulated that circulating levels of ferritin, also an acute phase reactant, are not truly reflective of body iron stores but may reveal other processes such as systemic inflammation. Evidence from experimental studies demonstrated that iron overload, including from hereditary or secondary hemochromatosis, can induce pancreatic beta-cell toxicity and impaired glucose metabolism. Although the exact molecular mechanism is not clear, iron is a strong pro-oxidant, and higher than normal iron concentrations may lead to diminished insulin secretion coupled with pregnancy-induced insulin resistance, thereby predisposing to GDM.6
MATERIALS AMD METHODS
A prospective study was carried out from October 2018 to December 2019 in the Department of Obstetrics and Gynaecology and Department of Pathology JN Medical College, AMU, Aligarh. Pregnant women with gestational age from 24-28 weeks were eligible for the study, the DIPSI OGTT was performed with a single dose of 75-gram oral glucose in non-fasting state. 2 ml blood was collected after 2 hrs in sodium fluoride vial and blood sugar was measured spectrophotometrically (semi auto analyzer).
Based on DIPSI 2hr blood sugar screening results, 35 GDM patients (having sugar > 140 mg/dl) and 15 controls (having sugar < 120 mg/dl) were selected, with few exclusion criteria.
Inclusion criteria-
Exclusion Criteria-
-
Women with overt diabetes.
-
Women with iron deficiency anaemia.
-
Women with acute or chronic liver disease or kidney disease.
-
Women with any acute or chronic inflammatory disease.
Sample Collection
Post DIPSI screening (after 1 week) the blood samples were collected from both the GDM and control groups. The HbA1C was measured with 2 ml EDTA blood using HPLC method on the same day. For serum Ferritin estimation an additional 2 ml blood samples were taken in the plane vial and serum was separated immediately and in case of any undue delay was stored refrigerated at, -20Degree Celsius till assayed and the test was performed by Ferritin ELISA kit.
Statistical Analysis
The data were analyzed using the SPSS 21 software. The data results were expressed as mean ±SD with mean differences and 95% confidence intervals. Student’s t-test was applied to compare data of cases and controls. Also, we applied Welch correction where the assumption of equal variances was violated.
RESULTS
The mean±SD of maternal age (yrs) in the participants was 27.6 ± 3.2 in GDM group and 27.3 ± 2.8 in the control group, Table 2 shows that the age (yrs) doesn't shows any statistical significance (p > 0.05) in the GDM and the control groups. The mean ±SD of initial DIPSI 2 hr blood glucose (mg/dl) was 175.9 ± 18.1 in GDM group and 84.0 ± 3.79 in the control group, Table 2 shows that the 2 hr blood glucose shows the significant higher value (p<0.05) in the GDM group as compared to the control group. The mean±SD of HbA1c (%) was 6.3 ± 0.4 in GDM group and 5.1 ± 0.2 in the control group, Table 2 shows that the HbA1c (%) shows the significant higher value (p<0.05) in the GDM group as compared to the control group. The mean ±SD of serum ferritin (ng/dl) was 79.5 ± 35.3 in GDM group and 32.9 ± 4.53 in the control group, Table 2 shows that the serum ferritin (ng/dl) shows the significant higher value (p<0.05) in the GDM group as compared to the control group.
DISCUSSION
The age of the participants had no significant difference in the GDM and control groups. The initial step DIPSI 2 hr blood glucose (mg/dl) showed significant difference (p< 0.05) in the GDM and control group, similar findings was seen in other studies using the DIPSI criteria for the diagnosis of GDM.7 In the subsequent evaluation of the cases, values of HbA1c (%) were significantly higher in GDM group 6.3 ± 0.4 as compared to the control group 5.1 ± 0.2, in a similar study, it was shown that HbA1c values were 5.5 ± 0.5% and 5.1 ± 0.4% for pregnant women with and without GDM respectively (p<0.001).8 In the present study serum ferritin (ng/dl) was 79.5 ± 35.3 in GDM group and 32.9 ± 4.53 in the control group (p<0.05), and similar findings were observed in a recent study which showed a value of serum ferritin 38.1± 4.6 in the GDM group and 33.5 ± 2.7 in the control group (p< 0.001).9 Studies on evaluation of serum ferritin in early pregnancy and the risk of GDM are few, but serum ferritin offers as a biomolecule having an important role in the development and progression of GDM. Iron overload has now significantly been associated with risk of both type 2 diabetes as well as GDM.10,11 The precise molecular mechanism by which this excessive iron contributes to diabetes has yet not been fully elucidated, however, the possibility which attracts attention is iron being a redox-active transitional metal having a powerful pro-oxidant activity. The iron-dependent Fenton reactions produce in-vivo reactive oxygen species (ROS) which are capable of disrupting the signalling of insulin in the liver and skeletal muscle while also causing damage to the pancreatic β cells.12 Given minimal in-vivo antioxidant defence mechanisms, the pancreatic β cells are especially susceptible to oxidative damage. In addition to the pro-oxidant effects of iron,, some studies have also suggested that high C-reactive Protein CRP (a biomarker of inflammation) level was also associated with GDM.13 Hence, the development and progression of diabetes mellitus might be related to inflammatory mechanisms. Similarly, some studies also found that ferritin–inflammatory–GDM mechanism is the basic pathophysiology in the development of GDM.14 It was considered that ferritin is a known acute?phase response protein, which increases in inflammation and therefore, high?level of ferritin leads to a sub-clinical generalized inflammatory state predisposing to GDM.15 However, it has been shown that high CRP is associated with GDM risk due to BMI as an intermediate factor.16 Hence, their on-induced oxidative damage model seems to be the best explanation of ferritin induced Diabetes Mellitus and GDM, however further studies are very necessary to explore the exact molecular mechanism.
CONCLUSION
High serum ferritin levels in GDM signify oxidative stress due to increased ROS production caused by iron overload. Therefore, it seems reasonable that anti-oxidants could play a significant role in the primary prevention and treatment of GDM.
Conflict of Interest: None
Source of Funding: None
References:
-
Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, et al. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: Phase I results of the Indian Council of Medical Research-India DIABetes (ICMR-INDIAB) study. Diabetologia 2011;54 (12):3022–3027.
-
American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018;41(1): S13–S27.
-
Mithal A, Bansal B, Kalra S. Gestational diabetes in India: Science and society. Ind J Endocrinol Metabol 2015;19(6):701-702.
-
Ren Y, Tian H, Li X, Liang J, Zhao G. Elevated Serum Ferritin Concentrations in a Glucose-Impaired Population and Normal Glucose Tolerant First-Degree Relatives in Familial Type 2 Diabetic Pedigrees. Diabetes Care 2004; 27 (2):622-623.
-
Chan KK, Chan BC, Lam KF, Tam S, Lao TT. Iron supplement in pregnancy and development of gestational diabetes—a randomised placebo?controlled trial. BJOG 2009;116(6):789-797.
-
Bowers KA, Olsen SF, Bao W, Halldorsson TI, Strøm M, Zhang C. Plasma Concentrations of Ferritin in Early Pregnancy Are Associated with Risk of Gestational Diabetes Mellitus in Women in the Danish National Birth Cohort. J Nutr 2016;146(9):1756-1761.
-
Pikee S, Sakshi M, Aruna N. Screening and Diagnosis of Gestational Diabetes Mellitus: from Controversy to Consensus. Curr Res Diabetes Obestet J 2017; 2(5): 555600.
-
Renz PB, Cavagnolli G, Weinert LS, Silveiro SP, Camargo JL. HbA1c Test as a Tool in the Diagnosis of Gestational Diabetes Mellitus. PLoS One 2015;10(8):e0135989.
-
Chauhan P, Gogoi P, Tripathi S, Naik S. Association of maternal serum ferritin level in gestational diabetes mellitus and its effect on cord blood hemoglobin. Int J Contemp Med Res 2020;7(1):1-4.
-
Jiang R, Manson JE, Meigs JB, Ma J, Rifai N, Hu FB. Body iron stores about the risk of type 2 diabetes in apparently healthy women. JAMA 2004;291 (6):711–717.
-
Lao TT, Chan PL, Tam KF. Gestational diabetes mellitus in the last trimester - a feature of maternal iron excess? Diabet Med 2001;18(3):218-223.
-
Ellervik C, Birgens H, Mandrup-Poulsen T. Need for reclassification of diabetes secondary to iron overload in the ADA and WHO classifications. Diabetes Care 2014;37(6):e137-e138.
-
Qiu C, Zhang C, Gelaye B, Enquobahrie DA, Frederick IO, Williams MA. Gestational diabetes mellitus about maternal dietary heme iron and nonheme iron intake. Diabetes Care 2011;34(7):1564-1569.
-
Pantham P, Aye IL, Powell TL. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta 2015;36(7):709-715.
-
Nicolas G, Chauvet C, Viatte L, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anaemia, hypoxia, and inflammation. J Clin Invest 2002;110(7):1037-1044.
-
Wolf M, Sandler L, Hsu K, Vossen-Smirnakis K, Ecker JL, Thadhani R. First-trimester C-reactive protein and subsequent gestational diabetes. Diabetes Care 2003;26(3):819-824.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License