IJCRR - 13(1), January, 2021
Pages: 92-97
Date of Publication: 05-Jan-2021
Print Article
Download XML Download PDF
Relationship of Growth Factors with the Development of Iron Deficiency Anemia in Girls Aged 12-14 Years Old
Author: Shaira Atadjanova, Abdurayim Arzikulov, Barnokhon Inakova, Dilfuza Patidinova, Barnokhon Rabieva
Category: Healthcare
Abstract:Introduction: According to the data of epidemiological studies, the prevalence of IDA, even within the same country, is not the same, and largely depends on the ecological, industrial, climatic and geographical conditions of residence. The most vulnerable contingent for the development of IDA includes adolescent children, especially women.
Objective: The main task of this article was to study the influence of growth factors in girls aged 12-14 years on the formation of iron deficiency anaemias, to determine ways to optimize their diagnosis. Methods: The intensity of the increase in length and body weight of girls, depending on the age and severity of ID is estimated. Also we measured, indicators of length and body weight of girls depending on the severity of iron deficiency and age.
Result: However, not all factors causing IDA at this age are equal. Judging by the literature data, the influence of growth factors on the ferrostatus of adolescents has been studied most poorly. Some authors believe that IDA negatively affects the anthropometry indicators of schoolchildren, increasing among them retarded (lagging) in physical and sexual development, while others indicate that increased growth and development are \"guilty\" of the manifestation of IDA, from - for the aggravation of the severity of latent iron deficiency \? LID.
Conclusion: Solving these issues would make it possible to concretize the methods of dispensary observation of adolescents with iron deficiency and develop more effective methods of prevention and therapy for the haemoglobin recovery of schoolchildren with IDA in its early stages.
Keywords: Iron deficiency anaemia, Latent deficiency anaemia, Ferrostatus, Adolescence, Haemoglobin, Physical development
Full Text:
INTRODUCTION
Epidemiological studies conducted in various regions of Uzbekistan have shown that the detectability of manifest ID in the form of IDA among the most vulnerable risk groups is impressive. At the same time, IDA is significantly widespread in risk groups in the regions of the Southern Aral Sea region, which is an area of ??ecological disadvantage.1,2 If we take into account that in all epidemiological studies as a screening method for detecting ID, an analysis of the haemoglobin (Hb) content in the blood is used, which allows only the manifest (explicit) ID to be identified, then it can be assumed that a large mass of the population suffering from latent (latent) forms JJ remains outside the field of vision of researchers.1,3 Therefore, it is quite clear that the true prevalence of ID is still unknown.4
The data of numerous studies on the identification of IDA among the population of Uzbekistan allow us to conclude that this region belongs to a high-risk group since the proportion of the manifest form of IDA exceeds 30% of the population, which corresponds to the critical level of the spread of the disease.5,6 These data require urgent measures to prevent IDA among the population, especially children.7 It was found that IDA is more often diagnosed in young children (up to 40%) and puberty-1/3 of adolescents.5,8 It is known that these age periods are characterized by an intensive growth rate, and adolescents - girls and even increased “loss” due to the onset of menarche.9,10 In these age periods, a large amount of iron is required, which is not always replenished by the food they consume.11,5
According to generalized world statistics, the prevalence of IDA in young children is from 8.2 to 39.5% 33, 344, in the Russian Federation (24.7-30%), and the incidence of LHD is from 22.4-41%.9,10
According to various authors, the incidence of IDA in children of our Republic varies from 17% to 62%, and there is an impression of the most widespread prevalence of this disease in Karakalpakstan, Surkhandarya, and Fergana Valley.4 In the Fergana Valley, the frequency of IDA among schoolchildren is still very high (up to 32%) and, unfortunately, does not tend to decrease.4,12 Children of senior school age, adolescents, conscripts, girls with the onset of the menstrual cycle are in a much worse situation in terms of the prevalence of IDA.
MATERIALS AND METHODS
The authors' studies indicate that in the Central Asian region in the development of IDA, the leading role belongs to unbalanced malnutrition, especially in children and adolescents, ie, during the period of increased iron intake to ensure growth and development.2
The unfavourable ecological situation is accompanied by a progressive deterioration in the state of health of the population, especially of children.2,8,11 In recent years, there have been works by domestic and foreign researchers to study the influence of anthropogenic environmental factors on the blood system.9 It has been established that each pathology has its own “elemental portrait”, reflecting the syndrome on which the given disease proceeds, and the participation of individual elements in its pathogenesis. The modern understanding of the essence of pathological processes is based on the recognition of the leading role of damage to the cell membrane.10 One of the mechanisms of damage to the cell membrane is lipid peroxidation (LPO).12 Even though LPO is currently being studied in many diseases, including anemia11, its significance in the pathogenesis of IDA is not fully understood.8 It has been shown that LPO-induced changes in the functional state of erythrocytes are characteristic of children with anaemia from regions of ecological disadvantage.13 It is emphasized that the indicators of erythrocyte viscosity (VE) and erythrocyte peroxide hemolysis (PGE), as well as an increase in LPO activity, a decrease in the antioxidant protection of erythrocyte membranes are factors in predicting the development of anaemic syndrome.2,13
The main task of this article was to study the influence of growth factors in girls aged 12-14 years on the formation of iron deficiency anaemias, to determine ways to optimize their diagnosis.
RESULTS AND DISCUSSION
Table 1 shows the main indicators of the physical development of girls aged 12-14 years, depending on the severity of iron deficiency. As can be seen from the data in Table 1, in girls aged 12-14 years with the development of LID, compared with the control group, there is an increase in body length (p <0.05), arm length (p <0.05) and legs (p <0.05), as well as the relative body surface - OPT (p <0.001) ( Table 1). Analyzing these data, it can be noted that the initial initiator of the accelerated growth of girls with LID in length is the tissue hypoxia factor (heme hypoxia has not yet developed). According to several researchers, two factors are responsible for the exchange of tissue iron: its reserves in the body and the erythropoietic activity of the bone marrow.2- It has been shown that hypoxic stress, activating the erythropoietic activity of macrophages in the bone marrow and kidneys, simultaneously increases the production of angiotensin II, prostaglandins E, J2, and adenosine2, as is known, are natural stimulators of the activation of the sympathetic division of the ANS, which in turn cause peripheral vasoconstriction and tachycardia.4 Although an increase in cardiac output is the primary compensatory response to a decrease in the oxygen-transporting capacity of arterial blood, changes in microcirculation can significantly affect oxygen transport at the tissue level.1,6
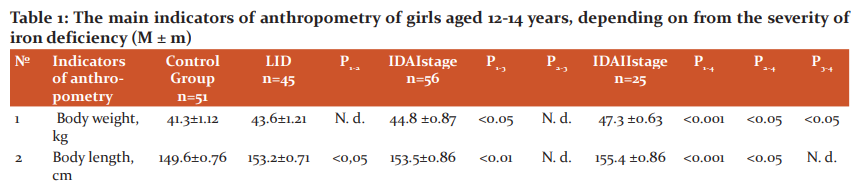
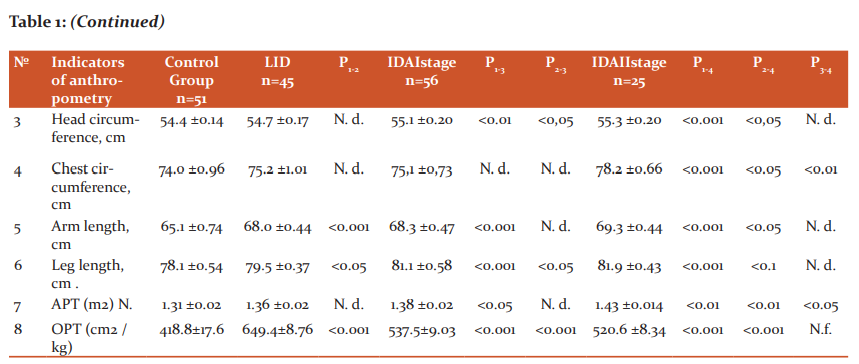
Note: 1. APT and OPT, respectively, absolute and relative body surface
2. N. d. - statistically insignificant (p> 0.05)
At the same time, we have revealed a phenomenon - an increase in the OPT of girls at the stage of LH, corresponds to the well-known surface law1,6, according to which the intensity of energy metabolism of homeothermic organisms proportionally increases with an increase in their relative body surface. It is known that the child's limbs, due to the presence of a shunt-countercurrent heat exchange system in them, play an important role in its growth in length and width, peripheral vasospasm contributes to increased growth in length (including limbs), and with vasodilation, there is an intensive growth in width and, accordingly, an increased increase in body weight and tissue differentiation. It should be borne in mind that a significant increase in body weight is accompanied by an increase in the number of laid nuclei of ossification, when the final formation of the main foci of medullary hematopoiesis occurs in the spongy substance of the skeleton, mainly in flat bones and vertebrae. We identified the morphometric situation in girls with LVH, characterized by intense growth in length, and combined with long arms and long legs, confirms the above physiological regularity with the only difference that the shifts occur at an earlier date (12-14 years), and not during the most intensive growth and maturation (15-17 years), ie, during the second puberty jump [8, 9]. As can be seen from the data in Table 1, in the surveyed girls with grade I IDA, along with high values ??of body length, arms, legs compared with the control group, body weight (p <0.05), head circumference, APT (p <0, 01), and OPT decreased compared with girls with LID (p <0.001).
With II severity of IDA in girls, the studied indicators of anthropometry are significantly increased compared to their peers in the control group and LID (p <0.001), but their differences when comparing I and II degrees of IDA become insignificant (p> 0.05), except for weight body (p <0.05), chest circumference (p <0.01) and APT (<0.05). Correlation analysis of serum iron content and bodyweight of girls (r = + 0.457 ± 0.02, p <0.05) and APT (r = + 0.427 ± 0.04, p <0.01) reveals a close positive relationship, and with body length - such a relationship was absent (r = + 0.285 ± 0.13, p> 0.05). Moreover, this relationship with increased weight gain (r = +0.619) and APT (r = +0.622) becomes closer. These data indicate that an increased increase in body weight and an increase in APT mediated by it is a compensatory morphometric response to preserve iron stores, which decreases with their increased growth in length.
In this regard, it seems to us that the premature, increased growth of girls in length (with LHD), like the accelerated course of the conveyor at the plant, may be accompanied by a greater probability of "collection errors" due to the dissonance of growth and differentiation of the girls' bodies and cause hypoxic « alterations.1,6,13 These factors in girls with IDA II severity may be the primary reasons for not realizing the maximum possible height in length (low growth in adulthood), due to the shortening of the period of puberty spurt (jump), lead to an earlier increased growth in width, because, body weight (their weight, chest circumference and APT are increased), which contributes to early puberty. In the literature, there are indications that the onset of the first menstruation is observed in girls upon reaching a certain body weight, regardless of the conditions in which they were.5
It has been shown that with the appearance of the first menstruation in girls, the intensity of growth in length sharply slows down.9
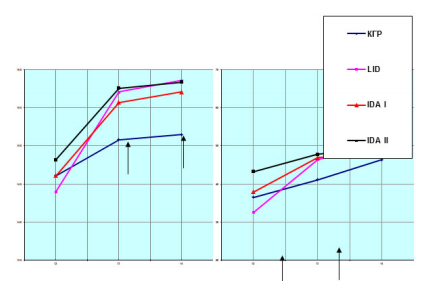
Figure 1: The intensity of the increase in length and body weight of girls, depending on the age and severity of ID. Indicators of length and body weight of girls depending on the severity of iron deficiency and age. Note: ↑ - designation, statistically significant periods (p <0.05-0.001)
From the data in Figure 1, It follows that in girls with ID, the intensity of weight gains and body length increases with age, which is similar for girls in the control group. However, their weight gains and body length gain occurs heterochromous, i.e., weight gain begins at earlier terms (12-13 years) than body length (13-14 years), while the intensity of these indicators is higher than their contemporaries in the control group.
The annual weight gains of girls in the control group at the age of 13 about the age of 12 was 12.6%, and at the age of 14 - 27.1%. These data in girls with LVD (42.6% and 54.3%), IDA I (23.7 and 26.6%), and II degree of severity (10.4% and 13.3%) are significantly changed. The key age was 13 years. At this age, the bodyweight of girls with LID, IDA of I and II degrees of severity was more by 13.1%, 14.1%, and 16.3% compared to the same age of girls in the control group.
The growth rate of girls in the control group at the age of 13-14 years compared with the age of 12 years was 3.22% and 3.69%, and in girls, with LID these data were 9.1% and 10.1%, IDA I stage (6.57% and 6.88%).
Consequently, in girls with PDJ, the most intense increase in body weight is observed, which is combined with their higher growth rates in length.
These data once again prove the above opinion that the premature accelerated growth of girls with PDJ leads to an accelerated weight gain, and the latter is the cause of their premature puberty. The literature shows that the highest growth rate (weight, length) is observed in the period preceding puberty,5,11 and some researchers10 consider the pubertal growth spurt as a period of realization of all the potential capabilities of the latter. It is interesting to note that the faster the growth spurt, the earlier the first menstruation occurs.9,10
During puberty, estrogens, together with androgens, are responsible for the maturation of skeletal bones and the appearance of secondary sexual characteristics, while estrogens have a more pronounced ability to stimulate maturation, and androgens - the growth of skeletal bones. In this regard, it can be assumed that at the stage of LVH, the influence of androgens on the sexual development of girls is more pronounced than estrogens.4,7,12
To reveal the mechanisms of the influence of growth factors on the development of ID in girls, you can use the index indicators of anthropometry (Table 2). It is known that the Brugsch and Erisman indices indicate age-related changes in the relationship between the chest circumference (BHC) and body length (TD).
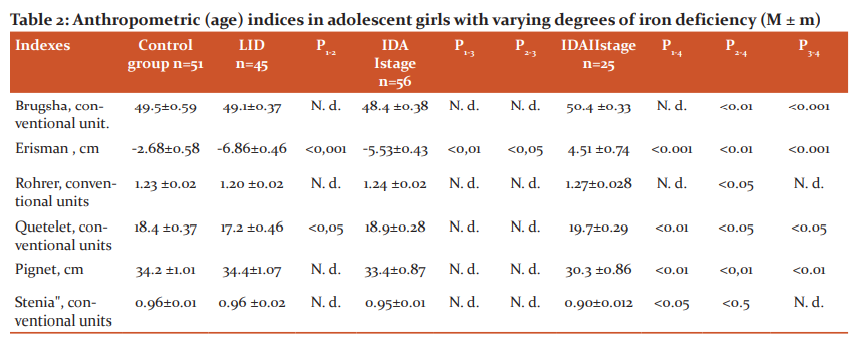
Note: 1. N. d. - statistically insignificant (p> 0.05)
As can be seen from the data table 2, in adolescent girls with the development of LID, due to their increased growth in length, the value of the negative Erisman index is aggravated (p <0.001).
This trend persists in adolescent girls with the development of grade I IDA, but it is less pronounced (p <0.05). The Erisman index for grade II IDA in the surveyed girls compared to the control group (p <0.001), LDL (p <0.001) and I degree of its severity (p <0.001) acquires a positive value, which indicates a slowdown in the rate of their growth in length and increased fat deposition in the chest.
This pattern is also confirmed by an increase in the Brugsch index in girls with IDA II severity of anaemia (p <0.001).
The Rohrer and Quetelet index show age-related shifts in the relationship between body weight (MT) and BM, their values ??decrease with increased height of children in length, and an intensive increase in MT increases these indicators [10, 11]. As evidenced by the data table. 4.2., In this regard, the Quetelet index turned out to be the most informative. This index with the development of LVD in girls aged 12-14 years is slightly reduced (p <0.05) and significantly increases with IDA II severity (p <0.01; p <0.05; p <0.05).
It is known that the Pignet and Stenia index indicates the relationship between MT, DT, and chest circumference (TBC) that occur during the growth and development of children. Table data. 2. indicates that in girls with the II severity of IDA, the values ??of the Pigment index (p <0.01, p <0.01, p <0.01) and "stenia" (p <0.05, p <0, 05, p> 0.05) are significantly reduced and indicate a slowdown in the growth rate of girls with ID in length, increased weight gain and fat deposition on the chest caused by it. This is evidenced by the close positive relationship of the parameters of the GCS in girls with MT (r = + 0.61 ± 0.09), rather than with TD (r = + 0.321 ± 0.10, p <0.05).
Thus, the results of the study of anthropometric indices, indicating age-related shifts in the relationship between MT, DT, and OGK, in girls aged 12-14 years with various degrees of ID are significantly changed. This is girls with the development of LID is manifested by increased growth in length, leading to a decrease in MT and fat deposition in the chest (Quetelet, Erisman index). With the development of manifest forms of ID (IDA), especially its II degree of severity in girls, and increased increase in MT (Quetelet index) is observed, and the latter is combined with an increase in fat deposition on the chest and a slowdown in the rate of their growth in length (Pignet index and Stenia). At the same time, the level of serum iron correlates positively with the Quetelet index (r = + 0.481 ± 0.14) and negatively with the Pignet index (r = -0.432 ± 0.11).
In the literature, there is information that with ID, first of all, its reserve fund (transferrin, hemosiderin) is consumed, and as the severity of ID increases, tissue iron is also lost. It seems to us that with the increased growth of children in length at the LV stage to replenish the ID, the reserve fund of iron is involved, as evidenced by the positive correlation between DT and the blood serum transferrin content (r = + 0.4114 ± 0.10, p <0.05), ie, the greater the height of children, the higher the serum transferrin content.6
CONCLUSION
We have found that a significant slowdown in the growth rate of adolescent girls with IDA I - II severity is aimed at reducing the consumption of the reserve fund and maintaining the level of its tissue pool. It is known from the literature that a slowdown in the growth rate of children in length and an increased increase in their weight is accompanied by differentiation of the cellular link and intensification of the mitochondrial apparatus of the liver, kidneys, and myocardium (cytochrome A, B, C) and usually coincides with an increase in the first menstruation of girls. We have no information on iron-containing enzymes (cytochrome oxidase, succinate dehydrogenase, catalase, etc.) and myoglobin in the blood of girls with varying severity of ID. However, the results of our studies indirectly indicate a decrease in iron at the tissue level in girls with IDA I and II stages, as evidenced by a significant decrease in the level of physical activity (see above), due to the increased weight gain and the resulting increased fat deposition in the chest.
ACKNOWLEDGMENT
The factor of hyperdynamic in schoolgirls-girls aged 12-14 years is "involved" in an increase in ID at the tissue level since a decrease in muscle activity is known - as a consequence of a decrease in cytochrome oxidase activity and myoglobin content in the blood by an excess of lactate and pyruvate. Also, in girls at this age stage of life, the proteolytic activity of the stomach (low hydrochloric acid secretion and pepsin secretion) is significantly reduced, actively participating in the assimilation of denatured myoglobin in the gastrointestinal tract with the participation of haemoxidases in further haemoglobin formation. These data once again prove the multicomponent and multifactorial nature of the causes of IDA in adolescent girls.
Conflict of interest: Authors declare that they have no conflict of interest.
Financial support: None
References:
1. Lavrisse M, Garsia-Casal MH, Mendez-Castellano H. Impact of fortification of flours with iron to reduce the prevalence of anaemia and iron deficiency among schoolchildren in Caracas, Venezuela: a Follow-up. Food Nutr Bull 2002;23(4):384-389.
2. Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 2005;353:1574–84.
3. Gordon N. Iron deficiency and intelligence. Paediatrics 2005;1:92-98.
4. Shankaran S, Laptook AR, Tyson JE, et al. Evolution of encephalopathy during whole-body hypothermia for neonatal hypoxic-ischemic encephalopathy. J Pediatr 2012; 160(4):567-572.e3.
5. Kara B, Cal S, Avdogan A, Sarper N. The prevalence of anaemia in adolescents: a study from Turkey. J Pediatr Hematol Oncol 2006;28(5):316-321.
6. Forman KR, Diab Y, Wong EC, Baumgart S, Luban NL, Massaro AN. Coagulopathy in newborns with hypoxic-ischemic encephalopathy (HIE) treated with therapeutic hypothermia: a retrospective case-control study. BMC Pediatr 2014;14:277.
7. Wyatt JS, Gluckman PD, Liu PY. Determinants of outcomes after head cooling for neonatal encephalopathy. Paediatrics 2007;119(5):912–21.
8. Gluckman PD, Wyatt J, Azzopardi DV, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: a multicenter randomized trial. Lancet 2005;365:663–70.
9. Zhou WH, Cheng GQ, Shao XM, et al. Selective head cooling with mild systemic hypothermia after neonatal hypoxic-ischemic encephalopathy: a multicenter randomized controlled trial in China. J Pediatr 2010;157:367–72.
10. Shankaran S, Pappas A, McDonald SA, et al. Predictive value of an early amplitude-integrated electroencephalogram and neurologic examination. Paediatrics 2011;128:e112–20.
11. Herbert N, Giabol MD, Suleymanova D, Gregory W, Evons MA. Anaemia in young children of the Muynak district of Karakalpakstan, Uzbekistan. Prevalence, Tyne and correlates. Am J Public Health 1998;88(5):147-148.
12. Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 2009;361:1349–58.
13. Felt B, Jimenez E, Smith J. Iron deficiency in infancy predicts altered serum prolactin response 10 years later. Pediatr Res 2006;60(5):513-517.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License