IJCRR - 13(1), January, 2021
Pages: 26-30
Date of Publication: 05-Jan-2021
Print Article
Download XML Download PDF
Effect of Tobacco Smoking on Salivary pH and Clinical Periodontal Indices in Indian Patients with Chronic Periodontitis
Author: Senthilkumaran M, Siji Jacob T, Asha J, Ravivarman C, Pradeep P Elango, Vijayalakshmi D
Category: Healthcare
Abstract:Introduction: Tobacco smoking has been known to assume the role of a robust risk factor for the progression of periodontal disease and salivary quantitation being an easily accessible tool can aid in assessing the diagnosis and prognosis of clinical parameters in smokers with chronic periodontitis.
Objective: Our study aims at the assessment of salivary pH and clinical periodontal parameters in Indian smoker patients diagnosed with chronic periodontitis.
Methods: In this study, 78 subjects between 25 years and 55 years of age were recorded. The habit history and clinical periodontal indices were recorded and subjects were divided into three groups: Group 1: 26 healthy subjects; Group 2: 26 non-smokers diagnosed with chronic periodontitis and Group 3: 26 smokers diagnosed with chronic periodontitis. The clinical periodontal indices recorded were Plaque index, Gingival Bleeding Index, Probing Pocket Depth (PPD) and Clinical Attachment Level (CAL). Salivary samples were collected and centrifuged and salivary pH is measured.
Results: The results indicated that group 3 had statistically significant higher plaque score level, clinical attachment loss and probing pocket depth measurements than group 2 and group 1. Group 2 had a higher gingival bleeding score than group 1and group 3 and the mean difference is statistically significant. Smokers with periodontitis had a decreased salivary pH (acidic) when compared to non-smokers and healthy subjects.
Conclusion: Cigarette smoking may be associated with a decrease in the salivary pH and variation of the various clinical periodontal parameters. Salivary samples can be used for early diagnosis of the severity of periodontitis and thus aid ineffective treatment.
Keywords: Smoking, Periodontitis, Salivary pH, Clinical periodontal parameters, Probing pocket depth, Gingival Bleeding Index
Full Text:
INTRODUCTION
Diseases of the periodontium continue to be one of man’s most widespread afflictions. Dental plaque is the chief etiological factor that explains the initiation and progression of gingival and periodontal disease.1 Despite plaque with pathogenic bacteria being the main aetiology, tobacco smoking assumes the role of a capable risk indicator contributing to the disease progression. Tobacco smoking greatly influences the structural and physical properties of the saliva. Also, salivary pH greatly influences the growth of oral microorganisms.2
Various studies on evaluation of salivary quantitation of pH in smokers3,4 and effect of smoking on periodontal tissues5,6 have been extensively undertaken previously, but the clinical periodontal parameters and salivary pH levels in smokers diagnosed with chronic periodontitis has been less studied. We aimed at evaluating the various clinical periodontal parameters and salivary pH in Indian smoker and non-smoker chronic periodontitis patients.
MATERIALS AND METHODS
A sample size of 78 subjects in the age group between 25 years and 55 years of age were segregated into 3 groups based on the inclusion, exclusion criteria, smoking history and clinical examination. A smoking history of 10 cigarettes per day for 2 consecutive years would sample the subject into the smokers' group. Subjects with a history of any co-morbidities
(Diabetes), chronic infections, or constant intake of any type of medication, any form of physical trauma in the last 2 weeks, or those with less than 22 permanent teeth were excluded from the study. The study also excluded patients diagnosed with aggressive periodontitis, acute periodontal conditions and patients with a history of smoking before two years.
All patients were briefly informed about the procedure and informed consent was taken. An extensive medical and smoking history (consumption and duration) was assessed by a standardized interview. Clinical examination was carried out utilizing the basic diagnostic instruments and William’s periodontal probe (Figure 1). The following clinical parameters were recorded: 1.Plaque index (Sillness and Loe 1964)7; 2. Gingival Bleeding Index (Ainamo and Bay 1975)8,9; 3. Probing Pocket Depth (PPD)9; 4. Clinical Attachment Level (CAL) (Ramfjord 1959).10
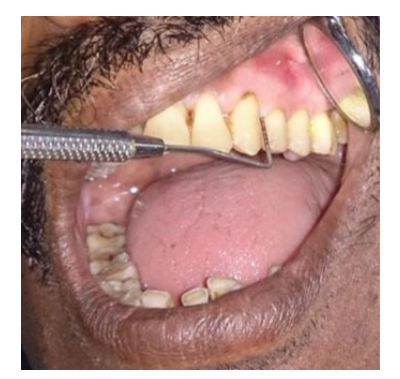
Figure 1: William's probe used for measuring the clinical periodontal parameters (Probing Pocket Depth being measured)
Smoking history and periodontal indices were taken into consideration to sort the subjects into three groups:- Group 1: included 26 systemically and periodontally healthy subjects with no loss of Clinical Attachment level and little or no Bleeding on Probing and Probing Pocket Depth less than 3mm; Group 2: Included 26 patients with no smoking history and clinically diagnosed as moderate to severe periodontitis (Clinical Attachment Loss more than 3 mm and Bleeding On Probing Probing Pocket Depth more than or equal to 5mm); Group 3: Included 26 patients with smoking history and clinically diagnosed as moderate to severe periodontitis.
Collection of Saliva sample
The saliva samples were collected in the morning on 2 hours fasting to avoid any stimulation. The subjects were restfully seated upright were and were instructed to flex their neck forward and gently spit out the saliva into the sterile test tube. The subjects were told not to spit forcibly to avoid blood contamination from an inflamed gingival tissue or an ulcerated lesion. About 5 ml of unstimulated saliva sample was collected and was centrifuged for 10 minutes at 3000 rpm.
Salivary pH estimation
A single electrode digital pH meter (ROYS instruments) was used to estimate the salivary pH levels. A pH tablet was then used for the calibration of the pH meter (pH 7 and pH 9.2). The procedure included immersing the single electrode in 0.1 N hydrochloric acid for 6 hours, followed by double distilled water. Sterile filter papers were then used to dry the electrodes before using it on the sample.11 All the data were analyzed using a software program (SPSS 11). Analysis of variance (ANOVA) and Scheffe multiple comparison tests were applied to collate the periodontal parameters among the groups.
RESULTS
Plaque Scores
Mean plaque scores in all the three groups were analyzed (Table 1). One way ANOVA test revealed that all the three groups were statistically different (p < 0.001). Scheffe multiple comparison tests have been applied to find out which of the three groups are statistically different. The results indicate that group 3 had a higher plaque score level than group 2 and group 1and the mean difference was statistically significant (p < 0.001). Group 3 had slightly higher plaque score level than group 2 and the mean difference was statistically significant (p < 0.001). In group1, mean plaque score was found to be 0.039 with a standard deviation of 0.034. In group 2, mean plaque score was found to be 1.415 with a standard deviation of 0.441. In the group 3, mean plaque score was found to be 1.913 with a standard deviation 0.571. One way ANOVA test revealed that all the three groups were statistically different (P < 0.001).
Scheffe multiple comparison test has been applied to find out which of the three groups are statistically different. The results indicate that group 3 has a higher plaque score level than group 2 and group 1 and the mean difference is statistically significant.(P < 0.001). Group 3 has slightly higher plaque score level than group 2 and the mean difference is statistically significant.( P < 0.001).
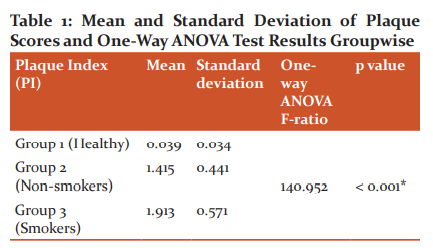
Gingival bleeding scores
One way ANOVA test and Scheffe multiple comparison test of the gingival bleeding scores (Table 2) indicated that group 2 had a higher gingival bleeding score than group 1 and group 3 and the mean difference was statistically significant (p < 0.001). However, Group 3 had slightly higher gingival bleeding score than group 1 and the mean difference was statistically insignificant (p =0.002). In group 1, mean gingival bleeding score was found to be 2.413% with a standard deviation of 1.773. In group 2, mean gingival bleeding score was found to be 89.093% with a standard deviation of 12.892. In group 3, mean gingival bleeding score was found to be 18.518% with a standard deviation of 23.924. One way ANOVA test revealed that all the three groups were statistically different (P < 0.001). Scheffe multiple comparison test has been applied to find out which of the three groups are statistically different. The results indicated that group 2 has a higher gingival bleeding score than group 1 and group 3 and the mean difference is statistically significant (P < 0.001) . Group 3 has slightly higher gingival bleeding score than group 1 and the mean difference is not statistically significant (P =0.002)
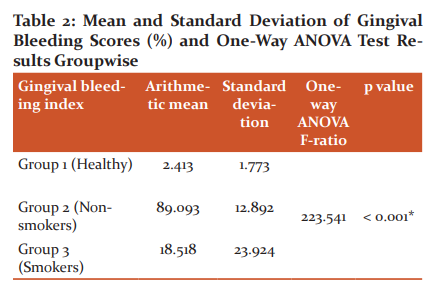
Attachment Loss values
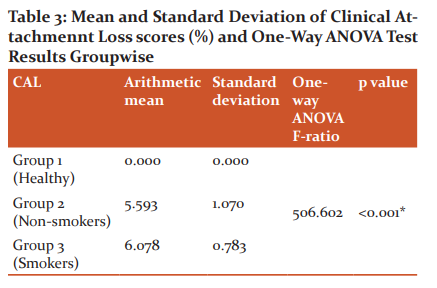
Clinical Attachment Loss values on analysis with one way ANOVA test indicated that group 3 had a higher Clinical Attachment Loss than group 1 and group 2 and the mean difference was statistically significant. Group 3 has slightly higher Clinical Attachment Loss than group 2 and the mean difference is not statistically significant (p=0.080). In Group 1, the mean clinical attachment loss was found to be 0.00mm with a standard deviation of 0.00. In group 2, mean clinical attachment loss was found to be 5.593mm with a standard deviation of 1.070. In group 3, mean clinical attachment loss was found to be 6.078mm with a standard deviation of 0.783. One way ANOVA test revealed that all the three groups were statistically different (P < 0.001).
Scheffe multiple comparison test has been applied to find out which of the three groups are statistically different. The results indicate that group 3 has a higher clinical attachment lossthan group 1 and group 2 and the mean difference is statistically significant (P <0.001). Group 3 has slightly higher clinical attachment lossthan group 2and the mean difference is not statistically significant (P=0.080).
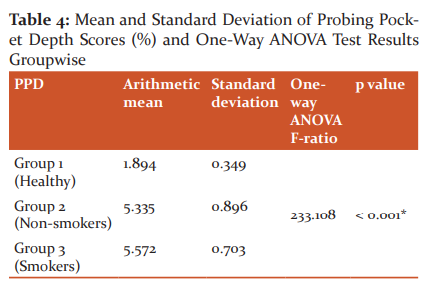
Probing pocket depth
The results of the probing pocket depth parameter indicated that group 3 had a higher Probing Pocket Depth than group 1 and group 2 and the mean difference was statistically significant (p < 0.001). Group 3 had slightly higher Probing Pocket Depth than group 2 and the mean difference is not statistically significant (p= 0.466). In Group 1, the probing pocket depth was found to be 1.894mm with a standard deviation of 0.349. In Group 2, the probing pocket depth was found to be 5.335mm with a standard deviation of 0.896. In Group 3, the probing pocket depth was found to be 5.572mm with a standard deviation of 0.703. One way ANOVA test revealed that all the three groups were statistically different (P < 0.001).
Scheffe multiple comparison test has been applied to find out which of the three groups are statistically different. The results indicate that group 3 has a higher probing pocket depththan group 1 and group 2 and the mean difference is statistically significant ( P< 0.001). Group 3 has slightly higher probing pocket depththan group 2 and the mean difference is not statistically significant (P= 0.466).
Salivary pH
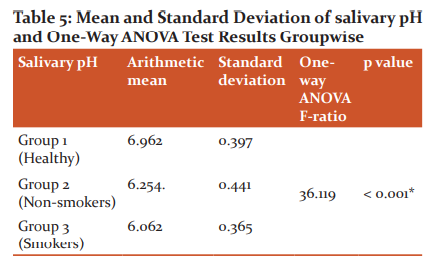
Group 1 had a higher salivary pH than group 2 and group 3 and the mean difference was statistically significant (p < 0.001). Group 2 had slightly higher salivary pH than group 3 and the mean difference was insignificant (p= 0.233). In Group 1, the mean salivary pH was found to be 6.962 with a standard deviation of 0.397. In Group 2, the mean salivary pH was found to be 6.254 with a standard deviation of 0.441. In Group 3, the mean salivary pH was found to be 6.602 with a standard deviation of 0.365. One way ANOVA test revealed that all the three groups were statistically different (P < 0.001). Scheffe multiple comparison test has been applied to find out which of the three groups are statistically different. The results indicate that group 1 has a higher salivary pH than group 2 and group 3 and the mean difference is statistically significant (P < 0.001). Group 2 has slightly higher salivary pH than group 3 and the mean difference is not statistically significant (P= 0.233).
DISCUSSION
Our study thus reveals that smokers had greater plaque accumulation than non-smokers and healthy subjects. This is in conformance with the studies done by Baumert et al.12 and Zuabi et al.13 where they observed that mean plaque scores were greater in smoking patients and that oral hygiene status was poorer in smokers compared to non-smokers. However, certain studies are showing an antithetical relationship between smoking and plaque accumulation. Studies done by Apatzidou et al.14 did not find statistically different plaque index among smokers compared with non-smokers.
Despite the significant increase in plaque scores among smokers compared to the non-smoking patients, the gingival bleeding was to be lesser in chronic periodontitis patients with smoking history and almost in par with the healthy subjects. This may be attributed to nicotine levels in cigarette smokers which is known to have vasoconstrictive effects. The thicker keratinization of the gingival mucosa may also account for the decrease in bleeding on probing in smokers.15
The significantly higher measures of Clinical Attachment Loss and Probing Pocket Depth in smokers despite the not so significant differences in plaque accumulation and gingival inflammation indicates a rise in progression and severity of destructive disease among smoking individuals diagnosed with chronic periodontitis.16 Normal pH of saliva ranges from 6.2 to 7.6 (average- 6.7). It is the salivary components that enable the maintenance of a neutral environment in the mouth essentially by the salivary flow and by the buffering components of saliva.Salivary diagnostics have been gaining increasing attention in recent times.17
A periodontally sound oral cavity and healthy dentition without dental caries usually present with a neutral ph of 7.0. If the saliva pH drops below 7.0 usually, an acidic oral environment prevails in the oral cavity, making it more prone to halitosis and chronic periodontitis. A less prevalent condition is a salivary pH greater than 7.0 that can also bring about the anaerobic micro-environment thus attributing to the disease severity.
Baliga et al. (2013)18 in a study comparing the salivary pH in healthy subjects, chronic gingivitis and chronic generalized periodontitis patients concluded that chronic generalized periodontitis patients had a lower salivary pH compared to the controls. Various studies with a few exceptions have also shown that most of the subgingival pathogens causing chronic periodontitis may be responsible for the acidic environment which further favours its growth. In some studies, it has been reported that smoking increases salivary flow in the short term and over time this lead to decreased salivary flow and an acidic pH and lower buffer capacity19. The outcomes of our study were found to coincide with the above study wherein smokers with periodontitis, the salivary pH was more acidic (pH - 6.062) compared to non-smokers (pH 6.254) with periodontitis and healthy subjects (pH 6.962).
CONCLUSION
Within the limits of our study, we find that salivary sample has the ease of being collected non-invasively and the salivary pH when correlated with the clinical periodontal parameters in both smoker and non-smokers with chronic periodontitis, plays an imperative role in predicting the severity of the periodontal disease.
Acknowledgement:
We acknowledge the immense help received from the scholars whose articles are cited and included in references to this manuscript. We are also grateful to authors/editors/publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
Conflict of Interest: None
Source of Funding: Nil
References:
-
Al-Tayeb D. The effects of smoking on the periodontal condition of young adult Saudi population. Egyptian Dental J 2008;54(1):15.
-
Singh M, IngleNA, Kaur N, Yadav P, Ingle E. Effect of long-term smoking on salivary flow rate and salivary ph. J Ind Assoc Public Health Dent 2015;13:11-3
-
Ahmadi-Motamayel F, Falsafi P, Goodarzi MT, Poorolajal J. Comparison of Salivary pH, Buffering Capacity and Alkaline Phosphatase in Smokers and Healthy Non-Smokers. Sultan Qaboos Univ Med J 2016; 16(3): e317–321.
-
McGuire JR, McQuade MJ, Rossmann JA, Garnick JJ, Sutherland DE, Scheidt MJ. Van Dyke TE. Cotinine in Saliva and Gingival Crevicular Fluid of Smokers With Periodontal Disease. J Period 1989; 60: 176-181.
-
Bergström J. Tobacco smoking and chronic destructive periodontal disease. Odontology 2004; 92:1–8.
-
Kinane DF, Chestnutt IG. Smoking and Periodontal Disease. Crit Rev Oral Biol Med 2000; 11(3):356-365.
-
John J. Textbook of community dentistry. 1st edition: 2003, CBS.
-
Wolf HF, Rateitschak EM, Hassell TM. Color Atlas of Dental Medicine: Periodontology. 3rd edition: 2005;309-19.
-
Newman MG, Takei H, Klokkevold PR, Carranza FA. Clinical periodontology. 8th Edition: WB Saunders.1996.
-
Newman MG, Takei H, Klokkevold PR, Carranza FA. Carranza’s Clinical Periodontology. 10th edition: WB Saunders.2006.
-
Vasudevan DM, Shreekumari S; Vaidyanathan K. Textbook of biochemistry for medical students. 6th edition ed: 2010.
-
Baumert Ah MK, Johnson GK, Kaldahl WB, Patil KD, Kalkwarf KL. . The effect of smoking on the response to periodontal therapy. J Clin Periodontol 1994; 21: 91–97.
-
Zuabi O, Machtei EE, Ben-Aryeh H, Ardekian L, Peled M, Laufer D. The effect of smoking and periodontal treatment on salivary composition in patients with established periodontitis. J Periodontol 1999;70(10):1240-1246.
-
Apatzidou D A, Riggio M P. and Kinane D F. Impact of smoking on the clinical, microbiological and immunological parameters of adult patients with periodontitis. J Clin Periodont 2005;32:973–983.
-
Ramli J. Taiyeb Ali T B Association between smoking and periodontal disease: A review of the literature. Ann Dentist Uni Malaya. 1999; 6:21-26.
-
Kinane DF, Peterson M. and Stathopoulou PG. Environmental and other modifying factors of the periodontal diseases. Periodontology 2006;40:107–119.
-
Rajasekar S, Ramasubramanian V, Sethupathi. Assessment of Salivary EnzymesalanineAminopeptidase(Alap) and Dipeptidyl Peptidase Iv (Dpp Iv) in Patients with Chronic Periodontitis. Int J Cur Res Rev 2016; 8(19): 6-11.
-
Baliga S, Muglikar S, Kale R. Salivary pH: A diagnostic biomarker. J Indian Soc Periodontol 2013;17(4):461-465.
-
Fenoll-PalomaresC, Montagud J, Sanchiz V, Herreros B, Benages A. Unstimulated salivary flow rate, pH and buffer capacity of saliva in healthy volunteers. Revista Espanola De Enfermmedades Digestivas 2004;96(11):773-783.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License