IJCRR - 8(6), March, 2016
Pages: 18-21
Date of Publication: 22-Mar-2016
Print Article
Download XML Download PDF
EVALUATION OF THE EFFECTS OF PH AND TEMPERATURE ON THE ADSORPTION ISOTHERM OF ACTIVATED MEDICOAL FROM NIGERIA PLANTS. MAGNIFERAINDICA, PERSEAGRATISSIMA AND PSIDIUMGUAJAVA
Author: Edwin N. Oguegbulu, Emmanuel A. Nwokeboth
Category: Healthcare
Abstract:Activated charcoal, also referred to as medicoal is known to be a versatile tool in the field of Pharmaceutical Medicine. This was successfully prepared from the stem parts of Mangiferaindica (Mango), Psidiumguajava (Guava) and Perseagratissima (Avagado pear) by process of carbonization and thermal activation. Liquid phase study was conducted using Freundlich isotherm model to evaluate the equilibrium adsorption data generated for methylene blue (MB) sulphanephthalein dye stuff on activated medicoal test samples. Ultra violet spectroscopic method was used to assess the effect of variation of pH and temperatures on the adsorption profile of all the medical samples, including the commercially available standard. The results were found to be statistically significant using the one-tail analysis of variance (ANOVA) at confidence interval of p ≤ 0.05. The research showed that the availability of negatively charged groups at the adsorbent surface is necessary for the adsorption of alkaline dye - stuff, thus the pH increase resulted to an increase in adsorption. The investigation also indicated that adsorption of locally sourced medicoal increases with temperature. The adsorption pattern of Perseagratissima compared favorably with that of the commercially available standard whereas Psidiumguajava ranked lowest.
Keywords: Locally sourced activated medicoal, Adsorption capacity, Function of, Temperature and pH
Full Text:
INTRODUCTION Adsorption is the adherence of atoms, ions or molecules from a gas, liquid or dispersed solid to surface Abrowski, A. D. (2005). By this, a film of the adsorbate is created in the surface of the adsorbent. Adsorption being distinct from absorption is a surface phenomenon whereas the later involves the whole volume of the material. There are two types of adsorption with their characteristic features and several applications in Pharmaceutical Medicine. These uses extend to control of odour, remedy for toxins and flatulence as well as in chromatographic techniques. Oguegbulu and Nwoke (2015).
Some physico- chemical factors are known to influence the adsorption performance of materials; Solids, particularly in finely divided state have large surface area and therefore, charcoal, silica gel, alumina gel, clay, colloids, metals of finely divided particles, all act as good adsorbents Transtutor.(2013). Usually, smaller particles equilibrate more easily and nearly full adsorption capacity canbe attained. Piero (2013). The high contact time between the adsorbent and the adsorbate creates more complete adsorption process. Zhu et.al (2012),so also, do the effects of the following impact on adsorption; pH, degree of ionization of molecules, temperature and the affinity of the solute for the adsorbent. Christmann.(2010/2011). Generally, pH has been found to affect the; activity, solubility, stability and absorption of medicinal products Niebergall(1980).
An increase in temperature implies that the molecules attract a quantum of heat energy equivalent to a product of the heat capacity and such infinitesimal increase in temperature. The associated increase in temperature alters the kinetic energy (Entropy) at the surface region thereby resulting in enhanced enthalpy which favours the surface phenomenon of adsorption.
At constant volume, this temperature effect can be expressed by the following transformation derivable from ClausiusClapeyron equation as: ? s = 2.303Cv log T2 /T1 Where; ? s is the change in entropy, Cv is the heat capacity at constant volume, where T1 and T2 are the limits of changes in thermodynamic temperatures. Florence and Attwood (1981). Three isotherm models are indentified, namely: Langmuir, Freundlich and Brunauer, Emmett-Teller (BET). Of these models, the Freundlich Isotherm model is the most appropriate for this study. Oguegbulu and Okumiahor (2013)
MATERIALS AND METHODS: The stem parts of Psidiumguajava (Guava), Perseagratissima (Avocado pear) and Margiferaindica (Mango) were harvested from Obelle village in Emoha Local Government Area of Rivers State in Nigeria. Each was chopped into chips, air dried for 28 days under the ambient laboratory temperatures. They were thereafter carbonized respectively using the Muffler furnace (Nabertherm, Germany)at 650o Cfor 30 minutes Fiyyaz et.al. (2000).
Each of the medicoal samples was collected, pulverized with mortar and pestle as well as sieved using mesh size number 250. The fine powdered samples so obtained were stored in air tight containers separately and labeled accordingly Ademiluyo et.al.(2009). The sieving process was also carried out for the commercial (standard) medical sample. The test samples were reactivated at a temperature of 100o C for 2 hrs prior to the experiments. This reactivation process helps to expel moisture and volatile oil impurities.Wikipedia. (2014). For the assessment of temperature effect, a 150 ml MB dyestuff solution was prepared with a concentration of 50mg/L and adsorbent dose of 2.0g/150ml.
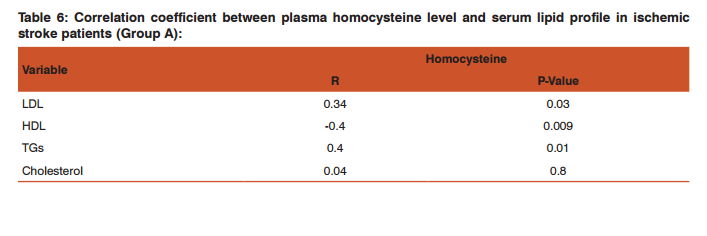
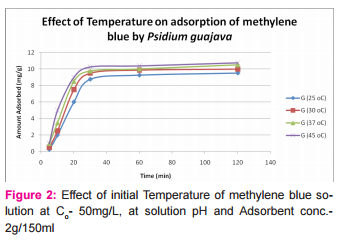
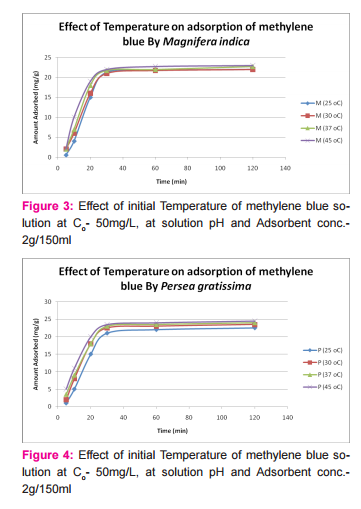
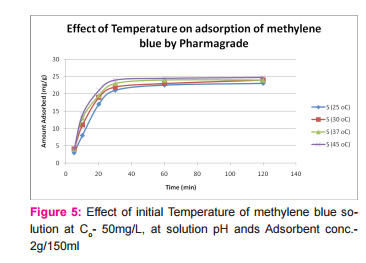
This was prepared by dissolving 7.5mg of MB in 150ml of distilled water in a conical flask and adding the 2.0g of activated medical to each. The mixtures were stirred to propel percolation and then subjected to different experimental temperatures as; 25, 30, 37 and 45o C in a water bath. Samples were collected from the mixtures, filtered and absorbance determined at different time intervals (5, 10, 20, 30, 60 and 120 minutes), for the different temperatures. Graphs of the amount adsorbed per unit mass of adsorbent (mg/g) against time (minutes) were plotted for each of the temperatures for various activated medicoal samples.
Effect of pH on the adsorption of MB on the test samples was conducted by preparation of five solutions as above. 150ml with concentrations of 50mg/L by dissolving 7.5 mg MB in 150ml of distilled water and their initial pH determined using pH meter(Mettler Toledo, Germany). The pH thereafter was adjusted by using 0.05N HCL for acidity and 0.05N KOH for alkalinity to obtain pH of 2, 5, 7, 9 and 12. To each of the various mixtures was added 2.0g of the various test medicoal samples. These were properly shaken and maintained at room temperature for 2 hrs. Following this, the samples were collected, filtered, the absorbance measured and concentrations extrapolated. A graph of amount adsorbed per unit mass of adsorbent (mg/g) versus corresponding pH of solutions was plotted. Hema, Martin, (2009).
DISCUSSION Results of comparative temperature and pH effects on the adsorption isotherm of developed medicoal samples as shown in figures 1 to 5 above, were used in the evaluation ofthe adsorption profiles of all the test medicoal samples. The study was conducted under the same experimental conditions such as, the particle size of adsorbent; uniform contact time; temperatures and pH. The effect of initial pH and temperatures on the adsorption of methyleneblue, dye stuffon medical was assessed by this research. At pH 2, adsorption was minimal, but was enhanced with an increase in the initial pH of the M.B dye solution. The adsorption of this cationic dye on the adsorbent surface was proportionately influenced by the surface charges on the adsorbent-medicoal samples, and is a function of the pH of the solution.
The results showed further that the availability of the negatively charged ions at the adsorbent surface is necessary for the adsorption of the MB dye and such was observable at pH above2. Adsorption was almost unlikely at that pH of 2 since there is a net positive charge in the adsorption system due to the presence of H3 O+ . Thus as the pH increases, more negatively charged surfaces are available, thereby facilitating a greater methylene blue adsorption. It was observed that adsorption increases with increasing pH in the case of all charcoal samples. This may be due to the fact that they all basically hadthe same composition and were activated using same activation method.The acidic stomach environment,will impact positively charged groups in the adsorbent surfaces and is a major condition for adsorption of an acidic adsorbates such as toxins that should exist in their unionized form there in. The reverse occurs in the basic pH region of the small intestine where basic adsorbates would be adsorbed more. The result of investigation of temperature effect on adsorption of medicoal samples showed that the adsorption of MB by the samples increased with increase in temperature.
This increase in adsorption may be as a result of increase in mobility of the large dye ions with temperature. An increasing number of adsorbate may also acquire sufficient energy to undergo an interaction with the active sides at the surface. Additionally, an increase in temperature may produce a swelling effect within the structure of the activated medicoal, thereby enabling large dye molecules to penetrate with ease and faster.. This temperature effect can as well be extrapolated to the human body environment of about 37o C as a useful phenomenon for maximal activity of medical in treatment. Thus in the case of pyrexia due to poisoning, the adsorption action of activated charcoal may be slightly enhanced which is a desirable effect for the poison victim.
CONCLUSION The adsorption Isotherm has highlighted the individual sample of Perseagratissima as ranking equally with the commercially available standard sample. It can then be established that the adsorption of MB on the various medicoal samples increased with an increase in both pH and temperature.
ACKNOWLEDGEMENT The authors are thankful to the entire staff members of Laboratories of Pharmaceutical and Medicinal Chemistry as well as Pharmacognosy and Physiotherapy Departments of University of Port Harcourt, Rivers State, Nigeria, for their co-operation during this research work. Much gratitude also should go to the Laboratory staff of National Agency for Food and Drug Administration and Control (NAFDAC), Port Harcourt Nigeria for their highly valued technical support in the realization of this research.
References:
1. Abrowski, A. D. (2005). Adsorption- from theory to practice, Advanced colloid interface science;pg 135 – 224.
2. Ademiluyo, F., Amadi, T., Nimisingha, J. (2009). Adsorption and Treatment of Organic Contaminants Using Activated Carbon from Waste Nigeria Bamboo, Rivers State University of Science and Technology, Port Harcourt, Nigeria, J. Appl. Sci. Environ. Manag.; 13(3): pp39 – 47.
3. Christmann, K. Adsorption. Lecture Series2010 /2011.”Modern Methods in Heterogenous Catalysis Research”. Institut Fur Chemie und Biochemie, Freie Universitat. Berlin.Pp. 1 -39.
4. Fiyyaz, A., Zill-i-hima, N., Waseem, S. (2000).Conversion of some Agro-Industrial Wastes into Useful Industrial Products. Pak. J. Agri. Sci.; 37: pp3 - 4.
5. Florence AT and Attwood D.(1981). Physicochemical Principles ln Pharmacy. Macmillan Ltd. N.y. Pp 66 -102.
6. Hema, M., Martin, D. (2009). Adsorption of malachite green onto carbon prepared from Borassus bark, The Arabian Journal for Science and Engineering;Vol. 34, Number 2A, July 2009.
7. NiebergallPJ(1980).Ionic solutions and Electrolytic Equilibria. InOsol Arthur (ed.).Remington’sPharmaceuticalSciencesMackP ublishing.Co.,Pennsylvania.Pp225-243.
8. Oguegbulu EN and Okumiahor J. Evaluation of the adsorption isotherm of activatedcharcoal used in pharmaceutical medicine from someNigerian plant parts, corn cobs, the wooden parts ofMangiferaindicaand Azandirachtaindica. Advancement in Medicinal Plant Research.Vol. 1(4), pp. 72-76, October 2013
9. Oguegbulu EN and Nwoke EA.(2015).Physicochemical and Spectroscopic Evaluation of Adsorption Potentials of Activated charcoal from stem parts of Mangiferaindica, Perseagratissima and Psidiumguajava for Pharmaceutical medicine. Novo Journal of Medical and Biological Sciences.Vol.4(2).2015.1-9.
10. Piero, M. A. Adsorption (last modified June 2005). Cited 8th November 2013 at 10:20am http://cpe.njit.edu/dlnotes/ CHE689/ Cls11-1.pdf
11. Transtutor. Surface area and adsorption (Last modified January, 2013). Cited 15th December 2013 at 1:15amhttp://www. transtutors.com/chemistry-homework-help/surfacechemistry/ adsorption.aspx
12. W.ikipedia (The Free Encyclopedia). Reactivation of Charcoal (Last Modified 17th November, 2012). Cited 9th January 2014 at 10am.http://en.m.wikipedia.org/wiki/Activated _carbon#section_7.
13. Zhu, J., Gao, J., Li, Y., Sun, T., Wu, S., (2012). In Preparation of Activated Carbons for SO2 Adsorption by CO2 and steam activation. J Twain Inst Chem Eng; 43(1): 112-119.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License