IJCRR - 8(8), April, 2016
Pages: 04-07
Date of Publication: 20-Apr-2016
Print Article
Download XML Download PDF
DETERMINATION OF BACTERIOCIDAL ACTIVITY OF BACTERIOCIN PRODUCED BY A CLINICAL ISOLATE OF E.COLI AGAINST HUMAN PATHOGENS
Author: Joseph Pushpa Innocent D., Margaret Theresa J., Premkumar D.
Category: Healthcare
Abstract:Aim: The aim of this study is to estimate the bactericidal activity of bacteriocin against the common human pathogens. A total of 62 specimens were collected from patients attending the department of surgery, Karpaga Vinayaga Institute of Medical sciences and Research Centre, during a period of six months. Methodology: Specimens collected were superficial pus swab from the wound, deep tissue biopsy and scraping from the base of the ulcers. Samples were cultured on Blood agar (BA) and MacConkey agar (MA). The plates were incubated at 37C over night. The aerobic organisms were identified by standard microbiological procedures as described in Bailey and scott's Daignostic Microbiology. (Finegold et al.1986).(1) The E coli which was isolated and identified was used in the preparation of crude bacteriocin.( 2) It was purified by Ammonium sulphate precipitation(3)and concentrated by centrifugation. Desalting of protein was done by dialysis method.(4) The protein concentrations of these purified bacteriocin was determined by Lowry's method.(5) Bacteriocin prepared from E coli was subjected for its bactericidal activity against some human pathogens by minimum inhibitory concentration method. Optical density (OD) of the culture was measured at 660 nm using spectrophotometer. Bactericidal activity of bacteriocin against human pathogens was estimated. Results: The results were analyzed, most of the pathogens need a very low concentration of the bacteriocin for the minimum inhibitory concentration (MIC).We observed that there is a slight variation in MIC among the different bacterial pathogens. Conclusion: In General when the concentration of the bacteriocin increased then the OD values of the culture tubes were decreased. In conclusion Bacteriocin not only have the bacteriocidal activity but also dose dependent in different bacteria.
Keywords: Bacteriocin, Bactericidal activity, Microbial virulence
Full Text:
INTRODUCTION Pathogenic microbes produce disease in man is due to certain enzymes and toxic substances released by bacteria called virulence factors. Some chemicals released by the bacteria may cause self destruction are known as autolytic enzymes. Similarly some other biologically active compounds, produced by the bacteria may active against strains of other species or closely related species. This type of bactericidal agents produced by Gram negative bacteria are called bacteriocins.(6) Bacteriocins are ribosomally synthesized antimicrobial peptides.(7) Classification of bacteriocins based on molecular size, producer organisms, chemical structure and the mode of action. Various names are given to these agents such as colicin, pyocin and or marcescins etc. Bacteriocin affect the essential function of livings cells such as transcription, replication and cell wall biosynthesis.(8) They also act by forming membrane channels or pores that destroy the energy potential of sensitive cells.(9) However bacteriocin producing strains are resistant to their own product. It has been evaluated for intended use as mouth wash since it kills the bacteria which cause dental plaque. Hence it is safe to use as a natural alternative to antibiotics and chemical germicides. Keeping this in mind, it was planned to study the bactericidal activity of bacteriocin on some of the human pathogens. Informed consent was obtained from those who underwent the test procedure in this study. Ethics committee of Dr. MGR University approved this study.
MATERIALS AND METHODS A total of 62 specimens were collected from gulutial, thigh, knee, leg, foot, breast and umbilical areas which included the wound swabs, pus, tissue biopsy and scraping from ulcers. Samples were collected aseptically from patients attended the surgery department of Karpaga Vinayaga Institute of Medical science and Research Centre, Chinnakolambakkam. Samples collected were inoculated on to culture media and the organisms grown after incubation were identified by standard microbiological methods. Among the organisms isolated, the E. coli was the most commonly observed (10) The clinically isolated E.coli was used in the preparation of crude bacteriocin, because it is the common source of bacteriocin.(11) E. coli isolates had been grown on nutrient broth with 5% inoculums by batch fermentation at a pH of 6.2. The broth culture was incubated at 37c for 12 hours. For the separation of bacteriocin the culture was centrifuged at 8000 rpm for 20 minutes. Cell free supernatant was subjected to solid ammonium sulphate at pH 4 with gradual increase of 20 to 90% saturation and held for 3hours at 4c. The mixtures were centrifuged at 12000 rpm for 20 minutes. The precipitate was recovered and it was then suspended in 5ml of 50m.mol of sodium phosphate buffer at pH 7 followed by desalting of the protein by dialysis. Protein concentration was determined by lowry’s method and compared with the standard bovine serum albumin. Estimation of bactriocin activity The purified bacteriocin compound from E.coli was subjected for its bacteriocidal activity against commom human pathogens. Organisms used for the assay were Klebsiella spp, Salmonella typhi, staphylococcus aureus and corynebacterium. Estimation of MIC for the above culture of organisms, 0.5 ml of each log phase cultures were transferred in to test tubes contain nutrient broth of appropriate concentration of bacteriocin. 100µl/mL to 500µl/ml were prepared and added to 8ml of broth culture and were inoculated at 37c for 24hours. Minimum inhibitory concentration was estimated by measuring the optical density of the culture tubes at 660nm using spectrophotometer.
RESULTS Antibacterial sensitivity of bacteriocin against human pathogens were estimated and the results were tabulated .Table: 1 showed the turbidity measurements of klebsiella spp
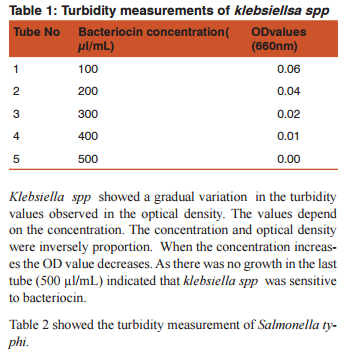
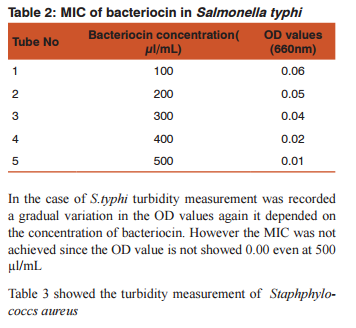
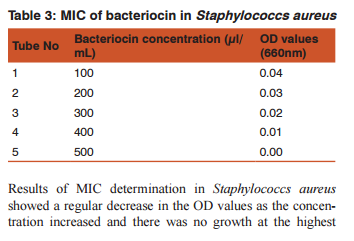
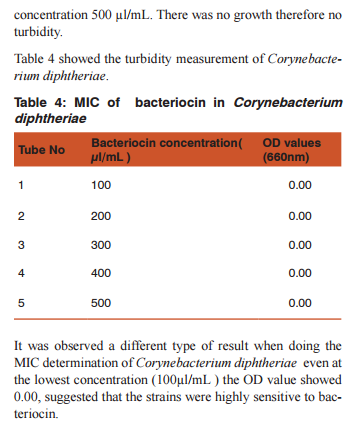
DISSCUSSION Bacteriocins are the subject of increasing attention as a major replacement for the conventional antibiotics and as natural food preservatives.(12) There are multiple bacteriocins present in the population of E.coli (13) and (14). Colicin production and the effect of growth inhibition was tested by solid methods.(15) In the present study turbidometry was used to assay the growth inhibition. In medicine the bacteriocins are important as they are produced by some non pathogenic bacteria act as commmonsal and loss of these bacteria following the use of antibiotics may cause the invasion of opportunistic pathogens. Hence it is decided to demonstrate the effect of bacteriocin on human pathogens. Further most of the mechanisms of bacteriocin in killing the bacteria are similar to that of antibiotics.(16)(17) Therefore it may replace the use of the antibiotics in the treatment. Hence this study was conducted as a step to determine the effect of bacteriocin on human pathogens.
CONCLUSION Though MIC studies of bacteriocin on human pathogens may not showed any consolidated report, but the results were encouraging. Present study results revealed that some strains of bacteria were sensitive to bacteriocin and are showed a response to its concentration. Each of the pathogen responded differently. Further studies are essential with still highly purified form of bacteriocin and the pathogens to be studied with wide range of bacteriocin concentration.
ACKNOWLEDGEMENT Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors/ editors/ publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
1. Finegold, S.M., Baron E.J. 1986. In. Bailey and Scott’s Diagnostic Microbiology. VII. Edn. Masby Company. St Louis. P. 106-125.
2. Yildirim. Z and Johnson M.G; ( 1998) Detection and characterization of a bacteriocin produced by Lactococcus lactis subsp. cremoris R isolated from radish,Latters in Applied Microbiology,26, 297-304.
3. Klaenhammer,T.R;(1998) Biochim, 70, 337-349.
4. Rajaram. G,Manivasagan.P,Thilagavathy .B, Saravanakumar .A.(2010) Purification and charactrization of a bacteriocin producied by Lactobacillus lactis isolated from marine environment. Advan J. food scie, and technology 2 (2) 138 – 144.
5. Lowry, O.H, Rosebrough .N.J, Farr A.L and Randall.R.J. (1951) Protein measurement with the folin –phenol reagent. J. Biol. chem.,193:265-275.
6. Gillis, R.R.,(1957) Colicin production as on epidemiological marker of Shigella sonnei,J.Hyg , 62, 1-9
7. Klienkauf ,H.V on Dohren, H.,(1990) Non ribosomal biosynthesis of peptide antibiotic, Eur ,J. bio chem. 192, 1-15.
8. Lakey,J.H. Gonzalezmanas,J.M.et al ., (1992) The membrane insertion of colicins, FEDS Lett, 307, 26-29.
9. Riley,M.A.,(1993) Positive secretion for colicin diversity in bacteria, Mol; Boil vol
10, 1048-1058. 10. Saleem M;Joseph Pushpa Innocent D.(2014) Comparison of microbial flora by deep tissue biopsy and superficial swab culture of specimen collected from various anatomical sites in early wound infections Int J Cur Res Rev, Vol 6.Issue 18, 24-28.
11. Shih-Chun yang, Chih- Hung Lin, Calvin T. Sung, and Jia-You Fang (20140) Antibacterial activities of bacteriocins: application in foods and pharmaceuticals Front Microbiol vol 5;241
12. Gillor O, Kirkup BC, Riley MA (2004) Colicins and Microcins the next generation antimicrobials. In Laskin BennettJW, M. Gadd G.Adv Appl. Microbiol Academic Press P 129-146
13. Margaret A. Riley, John E, Wertz (2002) Bacteriocin diversity: ecological and evolutionary prespecitves Biochimic 84-357- 364.
14. Maria Papagianni, Nicholaos Avramidis, George Filioussis, Despina Dasiou and loannis Ambrosiadis (2006) Determination of bacteriocin activity with bioassays carried out on solid and liquid substractes assessing the factor indicator Microorganism Microbial cell Factories 5: 30
15. David Smajs, Lenka Mioenkova, Jan Smarda, MartinVrba a(2010) Alena Sevcikova Zuzana valisova and Commensal Escherichia coli: colicin E1is a potential virulence factor BMC. Microbiology 10: 288.
16. Renee’ Pieterse; Svetoslav D.;Todorov (2010) Bacteriocins-exploring alternatives to antibiotics in mastitis treatment Braz J. Microbiol. Vol 41 no 3 Sao Paulo Oct 2010.
17. Cotter, P.D; Hill, C; Ross, R.P. (2005) Bacteriocins: Developing innate immunity for food. Nature Rev. Microbiology; 3;777- 788.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License