IJCRR - 9(7), April, 2017
Pages: 39-43
Date of Publication: 11-Apr-2017
Print Article
Download XML Download PDF
Prevalence and Risk Factors Associated with Coagulase-Negative Staphylococcus Infections in a Tertiary Care Center in North India
Author: Lubna Samad, Dalip K. Kakru, Bashir A. Fomda, Shugufta Roohi, Mohd Suhail Lone, Junaid Ahmad, Saalim Nazki, Nayeem-u-din Wani
Category: Healthcare
Abstract:Context: Coagulase negative Staphylococci (CoNS) are Gram positive cocci that are widespread commensals among mammalia. CoNS are more resistant to antimicrobials, including ß-lactam antibiotics, some hospitals revealing oxacillin resistance rates approaching 90%.
Aim: Determine the prevalence and antimicrobial susceptibility profile of CoNS in our hospital, and to observe various risk factors responsible for the isolation of clinically significant species.
Setting and Design: This prospective study was done in the Department of Microbiology, SKIMS, JandK over a period of 1year
Material and Methods: A total of 325 CoNS isolates were obtained from patients of all age groups and both the sexes. Antimicrobial susceptibility testing was done by Kirby-Bauer disc diffusion method. The minimum inhibitory concentration of vancomycin and teicoplanin for CSCoNS, was done by microbroth dilution method.
Statistical Analysis: The Chi-square test was used to compare two groups.
Results: Out of 325 CoNS recovered, 140 (43.1%) were found to be clinically significant. Maximum CSCoNS were isolated from the age group 0-9 years 27 (19.3%) and blood samples (n=48, 34.3%). Samples from the neonatal intensive care unit yielded the maximum number of CSCoNS, 29 (20.7%).
Hospital stay of >1 week, prior use of \?-lactam antibiotics and fluoroquinolones and intravenous line catheters were significant risk factors in patients from whom CSCoNS were recovered. Staphylococcus epidermidis was most common isolate. Methicillin resistance was seen in 79 (56.4%) of CSCoNS.
Conclusion: The recovery of CoNS should be seriously regarded as they are resistant to multiple antibiotics and their prevalence not only limits the treatment options but also acts as a reservoir of drug-resistant genes.
Keywords: Prevalence, CSCoNS- clinically significant CoNS, NSCoNS- non significant CoNS, Glycopeptide, Risk factors, MIC-minimum inhibitory concentration
Full Text:
Introduction
Coagulase negative Staphylococci (CoNS) are a heterogenous group of Gram positive cocci that are widespread commensals among mammalia.1 Unlike their coagulase positive counterpart, Staphylococcus aureus, CoNS produce a few virulence patterns and normally refrain from invading tissue.2 Staphylococcal biofilm formation is quite common in CoNS infections and markedly increases the MIC for older antimicrobials.1 Since CoNS are widespread on the human body and are capable of producing very large populations, distinguishing the etiologic agents from contaminating flora is a serious challenge, with the former confirmed only if the same strain is repeatedly isolated from a series of specimens.3
Staphylococcus epidermidis and Staphylococcus saprophyticus are the most frequently isolated clinically significant CoNS (CSCoNS) in clinical laboratories working with specimens of human origin with S. epidermidis alone accounting for 50-80% of the isolates, which are maximally nosocomially acquired.4 S. saprophyticus is a well-documented pathogen, colonizing the rectum or urogenital tracts of approximately 5% to 10% of women5 and is the second most common cause of uncomplicated cystitis in young healthy, sexually active women after Escherchia coli.2 S. haemolyticus has demanded increased interest recently because of the emergence of glycopeptide resistance.4 S. lugdunensis has been described to cause fulminant native valve endocarditis and prosthetic valve endocarditis.2,6,7
CoNS have historically been more resistant to antimicrobials, including ß-lactam antibiotics, than S. aureus and some hospitals reveal rates of oxacillin resistance in CoNS approaching 90%.1 The purpose of this study was to determine the prevalence and antimicrobial susceptibility profile of CoNS in our hospital, and to observe various risk factors responsible for the isolation of clinically significant species.
Materials and methods
A total number of 325 CoNS isolates were obtained from patients of all age groups and both the sexes, who were either admitted at SKIMS or attending its OPD. The clinical samples like blood, sputum, pus and other body fluids, urine, catheter tips received for routine culture and sensitivity between December 2012 to December 2013, were processed as per standard microbiological techniques for the recovery of bacterial pathogens.4, 8 Gram positive cocci that were catalase positive, slide and tube coagulase negative were identified as CoNS4, 8 and taken up for speciation by Vitek 2 compact. In case of urinary isolates from sexually active women, an additional Novobiocin disc susceptibility test was performed.4 CSCoNS,9 based on the repeated isolation from a single patient, clinical condition of the patient and the recovery of Staphylococcus saprophyticus from urine samples of reproductive age group females, were preserved for testing of glycopeptide resistance by microbroth dilution method.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing for penicillin, erythromycin, clindamycin, vancomycin, cotrimoxazole, tetracycline, ciprofloxacin, linezolid and nitrofurantoin (in urinary isolates only) was done by Kirby-Bauer disc diffusion method on Mueller Hinton agar plates. Methicillin resistance was screened by cefoxitin discs (30 µg/disc). The results were interpreted as per CLSI guidelines. 10
Minimum inhibitory concentration (MIC)
The minimum inhibitory concentration of vancomycin and teicoplanin for CSCoNS, was done by microbroth dilution method. Concentration of vancomycin and teicoplanin used was in the range of 0.125-64 µg/ml. Antimicrobial powders were obtained from HiMedia, Mumbai.
MIC endpoint was read as the lowest concentration of the antibiotic at which there was no visible growth. 10
Ethical Clearance
The ethical clearance was granted by the Institute’s Ethical Clearance Committee.
Statistical analysis
Data entry and analysis were done using SPSS Version SPSS 20.0. Percentages were calculated for categorical variables. The Chi-square test was used to compare two groups.
Results
A total of 1968 isolates of gram positive bacteria were recovered from patients admitted or attending the OPD at SKIMS, during the study period. Out of these, 325 (16.5%) isolates were found to be CoNS. From the 325 CoNS recovered, 140 (43.1%) isolates of CoNS were included in the present study in view of repeated isolation (two or more cultures) and the clinical condition of the patient thus found to be significant pathogens.
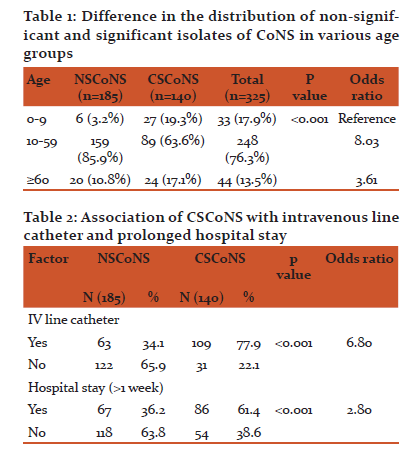
Maximum number of CSCoNS were isolated from patients in the age group of 0-9 years, 27 (19.3%) followed by the age group of ≥ 60 years, 24 (17.1%). Table 1
Maximum CSCoNS were isolated from blood, 48 (34.3%) followed by pus, 32 (22.9%); swab, 26 (18.6%); and ascitic fluid, 14 (10%). In addition to these 8 (5.7%) CSCoNS were recovered from catheter tips and from pleural fluid each. Least number of CSCoNS were recovered from urine 4 (2.9%).
Samples received from patients in neonatology/NICU, yielded the maximum number of CSCoNS 29 (20.7%) followed by general medicine, 27 (19.3%); general surgery 25 (17.9%); the surgical intensive care unit, 18 (12.9%); urology, 11 (7.9%); medical oncology 8 (5.7%) and endocrinology 8 (5.7%). In addition 5 (3.6%) isolates were recovered form neurosurgery, 4 (2.9%) from cardiology, 3 (2.1%) from gastroenterology and 2 (1.4%) from out-patient department.
All CSCoNS isolates were resistant to penicillin, 140 (100%). Methicillin resistance was seen in 79 (56.4%) isolates. Out of the 140 CSCoNS, 102 (72.9%) were resistant to Clindamycin, 121 (86.4%) were resistant to erythromycin and 118 (84.3%) were resistant to cotrimoxazole. Also 95 (67.9%) CSCoNS isolates were resistant to tetracycline and 126 (90%) were resistant to ciprofloxacin. None of the recovered isolates was resistant to vancomycin. However 4 (2.86), of the isolates of CSCoNS recovered were resistant to linezolid. Of all 4 CSCoNS recovered from urine, all (100%) were found to be resistant to nitrofurantoin.
Majority of the patients from whom CSCoNS were isolated had septicemia, 98 (70%); followed by abscesses, 21 (15%) with the most common species isolated being S. epidermidis, 67 (47.9%).
109 (77.9%) patients from whom CSCoNS were recovered had the presence of intravenous line catheters, whereas only 63 (34.1%) from whom NSCoNS (non significant CoNS) were recovered had IV line catheters, (p < 0.001); table 2. In addition, 86 (61.4%) patients with CSCoNS recovered had prolonged hospital stay (>1 week). (p < 0.001) Table 2
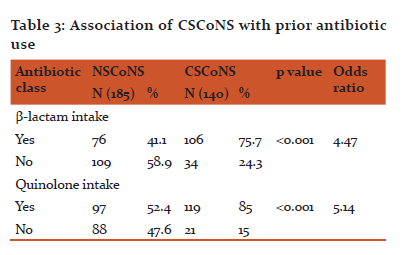
106 (75.7%) and 119 (85%) patients from whom CSCoNS were isolated had history of prior use of β-lactam antibiotics, and fluoroquinolones respectively. Table 3
MIC by microbroth dilution for vancomycin and teicoplanin was done on all the 140 CSCoNS isolates, which was found to be within the susceptible range for all of them.
Discussion
CoNS, because of their prevalence on human skin, mucous membranes and their relatively low virulence, have in the past been regarded as culture contaminants; however, in recent years, seen to be assuming greater importance as true pathogens.11,12 CoNS is the most commonly encountered organism in catheter-related bloodstream infections (CRBSIs), causing between 11%- 45% of infections with an incidence of 15.8 per 10,000 hospital admissions.13 In addition, a large proportion of nosocomial isolates of CoNS are resistant to multiple antibiotics, including penicillinase -resistant penicillins.11
At present, glycopeptides are among the last available antibiotics, for treating multidrug-resistant, gram-positive nosocomial infections, which are mostly caused by methicillin-resistant staphylococci and enterococci.14, 15
The prevalence of CoNS in our study was found to be 16.5% (325/1968), which is lower than reported in some studies such as Khadri H et al.16 (30.2% ) and Al-Mazroea AH et al. 17 (44.8%). Mir BA et al.18 from his study reported a CoNS prevalence of 14.3% from their hospital.
19.3% of CSCoNS were isolated from patients in the age group of 0-9 years, followed by the age group of ≥60 years (17.1%). Cercenado E et al.11 ,found that 38.5% of CSCoNS were from age-group of ≥60 years and 50-59 years each followed by 0-9 year age group (23.07%). This is consistent with our study as the most of the patients in these age groups are vulnerable to many infections especially when admitted in acute care settings such as the ICU’s of the hospital. Amita J et al. 19 reported CoNS to be the most common organisms associated with neonatal late-onset septicemia (>50%). In this study, an increased rate of isolation of CoNS was seen in 0-9 year age group, most of the patients being admitted in ICU’s for long periods. Placement of intravenous lines and the administration of high end antibiotics like third generation cephalosporins and carbapenems and prolonged ICU stay could have been instrumental in infections (due to CoNS) in these patients.
A significantly higher isolation of CSCoNS from blood (34.3%) was seen as majority of the patients had sepsis with bacteremia. Similar results were reported by Sharma V et al.20 and Begum SE et al.21 who in their studies isolated maximum number of CoNS from blood (46.33%, 48.6%, respectively).
Also 4 (2.9%) S. saprophyticus were isolated from urine. All the 4 isolates belonged to women in the age group of 30-39 years. Two of the four females were pregnant (1st trimester) and had urinary tract infection. The other two had pyuria.
Most of our CSCoNS were isolated from patients housed in high dependency areas; the neonatology/NICU (20.7%) of our hospital followed by the SICCU (12.9%). Tacconelli E et al.14 in their study found that admission to any ICU predisposes to infection with CoNS. Similarly Cimiotti JP et al.22 reported an increase in the rate of CoNS infection in NICU (34%) from their hospital.
Penicillin resistance was demonstrated in 100% of the CSCoNS with 56.4% of the isolates being methicillin resistant. A resistance of 72.9% was seen to clindamycin, 86.4% to erythromycin and 84.3% to cotrimoxazole. 67.9% and 90% of the CSCoNS were resistant to tetracycline and ciprofloxacin respectively. None of the recovered isolates was resistant to vancomycin. However 2.86% of the isolates recovered were resistant to linezolid. Out of the 4 isolates of CSCoNS recovered from urine, all (100%) were found to be resistant to nitrofurantoin. Similar results were seen by Al Mazroea et al.17 where the authors reported 99.24% resistance to penicillin in CoNS, followed by high resistance to erythromycin, cotrimoxazole and clindamycin. In agreement with our study, they found 100% vancomycin sensitivity in all CoNS isolates. Asangi SY et al.23 demonstrated a resistance of 16.7% to linezolid in their study. Antibiotic resistant CoNS has emerged as a major cause of morbidity and mortality in hospital setting in the last decade.24
The presence of intravenous line catheters was seen significantly higher in patients from whom CSCoNS were recovered (77.9%). In a study done among CRBSIs by Worthington T et al.,25 the authors found that 96% of the causative agents were CoNS. Our results are concordant with Al Mazroea AH et al.17 who documented that the prolonged use of intravascular catheters, in adult or neonatal ICUs predispose to bloodstream infection. In addition 61.4% of the patients from whom CSCoNS were recovered had prolonged hospital stay (>1 week) than those with NSCoNS (p < 0.001). These results are in agreement with many other studies conducted worldwide. 14, 17
Prior use of β-lactam antibiotics (75.7%) and fluoroquinolones (85%) was significantly higher in patients from whom CSCoNS were isolated. Tacconelli E et al.14 reported that patients with methicillin resistant CoNS (MRCoNS) bacteremia experienced significantly higher exposure to β-lactam antibiotics and cephalosporins. Mir BA et al.,18 reported that after exposure to multiple antibiotics due to their indiscriminate use, patients become colonized with multi-drug resistant CoNS strains which predisposes to infection.
Majority of CSCoNS were isolated from patients who had septicemia (70%). CoNS have been implicated as a significant cause of late-onset neonatal septicaemia as stated by Cimiotti JP et al.22
This study identified a total of 10 species of CoNS, with Staphylococcus epidermidis (47.9%) being isolated as the most common species followed by Staphylococcus haemolyticus, 28.6%. In a study by De Paulis A et al.26 ,the authors revealed 51 % strains to be S. epidermidis, followed by S. haemolyticus (18%) and S. saprophyticus (16%). Many other studies have reported Staphylococcus epidermidis to be the most common species of CoNS isolated.18, 20, 23, 27
MIC was done by microbroth method for vancomycin and teicoplanin on all the 140 CSCoNS isolates. However none of the isolates was found to be resistant to any of these antibiotics. The MIC for teicoplanin was slightly higher in our isolates as compared to vancomycin. In a study conducted by Center KJ et al.28 antimicrobial susceptibility testing of CoNS by microbroth dilution method revealed that 50 and 90% of the isolates were inhibited at a concentration of 1 and 2 µg/ml, respectively for vancomycin with the risk factors associated with higher vancomycin MIC being male gender, presence of a central venous catheter, prior exposure to any antibiotic including vancomycin and prolonged ICU stay.
Conclusion
Isolation of CoNS and their antibiotic susceptibility pattern should be regarded with all seriousness in clinical practice and clinical epidemiology because these are often missed as true sources of infection and their significance needs to be proved by repeated isolation from the samples. By being resistant to multiple antibiotics, (MRCoNS in particular), their prevalence in the hospitals not only limits the treatment options but also acts as a reservoir of drug-resistant genes.
Acknowledgement
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
Source of funding: None
Conflict of interest: None
References:
- John JF, Harvin AM. History and evolution of antibiotic resistance in coagulase-negative staphylococci: Susceptibility profiles of new anti-staphylococcal agents. Therapeutic and Clinical Risk Management. 2007; 3: 1143-52.
- Mandell GL, Bennett JE, Dolin R. Staphylococcus epidermidis and other coagulase-negative staphylococci. In: Principles and Practice of Infectious Diseases. 7th edn. Churchill Livingstone Elsevier; 2010: p. 2579-86.
- Sharma P, Lahiri KK, Kapila K. Conventional and molecular characterization of coagulase-negative staphylococcus in hospital isolates. IJPM; 54: 85-9.
- Koneman EW, Allen SD, Janda WM, Schreckenberger PC, Winn WC jr. Gram-positive cocci Part I: Staphylococci and related gram-positive cocci. In: Color Atlas and Textbook of Diagnostic Microbiology. 6th edn. Lippincott Williams and Wilkins. 2006; p. 624-651.
- Rupp ME, Soper DE, Archer GL. Colonization of the female genital tract with Staphylococcus saprophyticus. J Clin Microbiol. 1992; 30: 2975-9.
- Herchline TE, Ayers LW. Occurrence of Staphylococcus lugdunensis in consecutive clinical cultures and relationship of isolation to infection. J Clin Microbiol. 1991; 29: 419-21.
- Sharma P, Lahiri KK, Kapila K. Conventional and molecular characterization of coagulase-negative staphylococcus in hospital isolates. IJPM; 54: 85-9.
- Cunha MLRS, Sinzato YK, Silveira LVA. Comparison of methods for the identification of coagulase-negative staphylococci. Mem Inst Oswaldo Cruz. 2004; 99: 855-60.
- Horan TC, Andrus M, Dudeck. CDC/NHSN surveillance definition of health-care associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008; 36: 309-32.
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informational Supplement. CLSI document M100-S23. Wayne, PA: Clinical and Laboratory Standards Institute; 2013.
- Cercenado E, Garcia-Leoni ME, Diaz MD, Sanchez-Carrillo C, Catalan P, Bernaldo JCL. Emergence of teicoplanin-resistant coagulase negative staphylococci. J Clin Microbiol. 1996; 34: 1765-8.
- Pfaller MA, Herwaldt LA. Laboratory, clinical, and epidemiological aspects of coagulase-negaive staphylococci. Clin Microbiol Rev. 1988; 1: 281-99.
- Rewa O, Muscedere J, Reynolds S, Jiang X, Heyland DK. Coagulase-negative Staphylococcus, catheter-related, bloodstream infections and their association with acute phase markers of inflammation in the intensive care unit: An observational study. Can J Infect Dis Med Microbiol. 2012; 23: 204-8.
- Tacconelli E, Tumbarello M, Donati KG, Bettio M, Spanu T, Leone F, Glycopeptide resistance among coagulase-negative staphylococci that cause bacteremia, Epidemiological and clinical findings from a case-control study. Clin Infect Dis. 2001 Nov 15; 33: 1628.
- Sieradzki K, Villari P, Tomasz A. Decreased susceptibilities to teicoplanin and vancomycin among coagulase-negative methicillin-resistant clinical isolates of staphylococci. Antimicrob. Agents Chemother. 1998; 42: 100–07.
- Khadri H, Alzohairy M. Prevalence and antibiotic susceptibility pattern of methicillin-resistant and coagulase-negative staphylococci in a tertiary care hospital in India. Int J Med Med Sci. 2010; 2: 116-20.
- Al-Mazroea AH. Incidence and clinical significance of coagulase negative staphylococci in blood. J T U Med Sci. 2009; 4: 137-47.
- Mir BA, Srikanth D. Prevalence and antimicrobial susceptibility of methicillin resistant Staphyloccus aureus and coagulase-negative staphylococci in a tertiary care hospital. Asian J Pharm Clin Res. 2013; 6: 231-34.
- Amita J, Agarwal J, Bansal S. Prevalence of methicillin-resistant, coagulase-negative staphylococci in neonatal intensive care units: findings from a tertiary care hospital in India. J Med Microbiol. 2004; 53: 941-44.
- Sharma V, Jindal N, Devi P. Prevalence of methicillin resistant coagulase negative staphylococci in a tertiary care hospital. Iran J Microbiol. 2010; 2: 185-88.
- Begum ES, Dr. Anbumani N, Dr. Kalyani J, Dr. Mallika M. Prevalence and antimicrobial susceptibility pattern of coagulase-negative staphylococcus. Int J Med Public health. 2011; 1: 59-62.
- Cimiotti JP, Haas JP, Latta PD, Wu F, Salman L, Larson EL. Prevalence and clinical relevance of Staphylococcus warneri in neonatal intensive care unit. Infection Control Hosp Epidemiol. 2007; 28: 326-30.
- Asangi SY, Mariraj J, Satyanarayan MS, Nagabhushan, Rashmi. Speciation of clinically significant coagulase negative staphylococci and their antibiotic resistant patterns in a tertiary care hospital. Int J Biol Med Res. 2011; 2: 735-9.
- Mohan V, Jindal N, Aggarwal P. Species distribution and antibiotic sensitivity pattern of coagulase negative staphylococci isolated from various clinical specimens. Ind J Med Microbiol. 2002; 20: 45-46.
- Worthington T, Lambert PA, Elliot TS. Is hospital-acquired intravascular catheter-related sepsis associated with outbreak strains of coagulase negative staphylococci. J Hos Infect. 2000; 46: 130-34.
- De Paulis A, Predari S, Chazarreta C, Santoiani J. Five-test simple scheme for species level identification of clinically significant coagulase negative staphylococci. J Clin Microbiol. 2003; 41: 1219-24.
- Oliveira AD, Sanches P, Lyra JC, Bentlin MR, Rugolo LMSS, Cunha MLRS. Clinical Medicine Insights: Pediatrics. 2012; 6:1-9.
- Center KJ, Reboli AC, Hubler R, Rodgers GL, Long SL. Decreased vancomycin susceptibility of coagulase-negative staphylococci in a neonatal intensive care unit, evidence of spread of Staphylococcus warneri. J Clin Microbiol. 2003; 41: 4660-5.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License