IJCRR - 10(17), September, 2018
Pages: 11-16
Date of Publication: 10-Sep-2018
Print Article
Download XML Download PDF
Formulation of Okra-Based Antidiabetic Nutraceutical from Abelmoschus esculentus (L.) Moench (Ex-maradi Variety) and Evaluation of its Effect on Alloxan-induced Diabetic Rats
Author: Matazu K.I., Muhammad I., Bilbis L.S., Abbas A.Y.
Category: Life Sciences
Abstract:Following our report on the development of okra-based antidiabetic nutraceutical formulation and the establishment that 10:90 % (Seeds: Peels) is the formulation with optimal antidiabetic and antioxidant properties in-vitro; this study evaluated in-vivo antidiabetic effects of the developed formulation in alloxan induced diabetic rats. Diabetes was induced by intra-peritoneal administration of a single dose (150 mg/kg body weight) of Alloxan. The rats were randomly divided into five groups of six rats each. The Normal Control (NC) and the Diabetic Control (DC) groups were orally treated with normal saline (10 ml/kg); the Metformin Control (MC) group was orally treated with Metformin (100 mg/kg) while the Test groups (FX1) and (FX2) were orally treated with 100 and 200 mg/Kg body weight of the formulation respectively. All the groups were treated for 21 days. The effects of the treatments on blood glucose level, glycated hemoglobin and lipid profile parameters were studied for the antidiabetic evaluation. Administration of FX1 and FX2 to the respective test groups for 21 days resulted in significant (P< 0.05) reduction in blood glucose level, glycated hemoglobin and improvement on Lipid profile compared with the diabetic control (DC) group. Based on these findings, the study demonstrated the efficacy of the Okra-based nutraceutical formulation as a potent antidiabetic formula.
Keywords: Abelmoschus esculentus, Diabetes Mellitus, Glycated hemoglobin, Lipid profile, Nutraceutical formulation
Full Text:
INTRODUCTION
Diabetes mellitus (DM) is a widely spread epidemic disease and a serious metabolic disorder of carbohydrate, fat and protein metabolism reflected by an inappropriate high blood glucose levels (hyperglycemia) which results from the absence of insulin (Type 1 DM), decreased secretion (insufficient) of insulin and/or impaired (inefficient) action of insulin (Type 2 DM) [1,2]. Pancreas plays important role in the regulation of blood glucose level; it mainly consists of four types of cells viz: Alpha cells which secrets glucagon; Beta cells which secrets insulin; Delta cells which secrets somatostatin and Gamma cells which secrets pancreatic polypeptide [3]. The increased level of blood glucose stimulates insulin secretion from the Beta cells of the Pancreas while Alpha cells’ secrets Glucagon in the condition of low blood glucose level, to maintain the normal blood glucose level in the body. The imbalance between insulin and glucagon is one of the major factors in the pathogenesis of diabetes mellitus [3]. DM is currently treated / managed by different types of synthetic oral hypoglycemic agents such as Biguinides, Sulfonylureas, Thiazolidinediones α- glucosidase inhibitors and or insulin injection [4,5]. These are associated with several side effects and their efficacies are sometimes debatable [5]. Hence, attention has been directed towards alternatives and of which is nutraceuticals originating from food plants that are rich in antidiabetic phyto-constituents [6]. Also, recent efforts for the complementary treatment of diabetes have focused on functional foods and their bioactive compounds [7]. According to World Health Organization (WHO), more than 80% of the world’s population relies on traditional medicine for their primary healthcare needs [8]. Bioactive chemical substances in plants such as alkaloids, phenols flavonoids, glycosides, gum, polysaccharides, peptidoglycans, guanidine, steroids, triterpenes, terpenoides, carbohydrates, glycopeptides, amino acids and inorganic ions are responsible for their medicinal value [9]. However, few anti-diabetic poly-herbal products / formulations that contain plants metabolites as the active ingredients have been developed. For example, Alangium salvifolium tablet extracted from Alangium salvifolium and Gycin max [10]; Ipomea digitata tablet extracted from Ipomea digitata [11]; Bitter gourd tablets extracted from Momordica charantia [12]; Diamed powder extracted from Azardirachta indica, Cassia auriculata and Momordica charantia [13,14]; Also, Polyherbal product extracted from green tea have been documented and are commercially available [3]. Due to the perceived effectiveness, less side effects in clinical experience and relatively low costs of herbs; herbal drugs are becoming more popular as an antidiabetic agents [15].
Abelmoschus esculentus L. (Okra) is a popular health food due to its nutritional and health values [16]. It is a flowering plant that belongs to Malvaceae family. It is valued for its edible green pods and seeds. A number of previous studies have reported that Abelmoschus esculentus (Okra fruit) possessed hypoglycemic effect [17,18,19,20]. Ex- maradi Okra fruit; (a commercially/ locally available dry okra fruit characterized by its viscous nature) have also been reported to have significant antidiabetic activity [1]. Attempt have been made to improve the acclaimed hypoglycemic effect of the okra fruit by formulating varying proportions of the seeds and peels of Ex- maradi Okra fruit in the ratio: (10:90, 20:80; 30:70; 40:60, 50:50 and vice versa); then, the antidiabetic and antioxidant potentials of the varying proportions were tested in-vitro. The 10:90 % (seeds: peels) ratio was observed to be the most potent as it shows optimal in-vitro antidiabetic and antioxidant effect [1]. Hence the aim of this research work is to evaluate in- vivo, the antidiabetic effect of this [10:90 % (seeds: peels)] nutraceutical formulation.
MATERIALS AND METHODS
Chemicals and Reagents:
Analytical grade laboratory chemicals and reagents were used for this study.
Okra Sample Collection:
Ex-maradi (a commercially available Okra fruits from the vegetable growers/sellers at Maradi, Niger) was obtained from Maggi market at Sokoto State, Nigeria. The sample was identified and authenticated by Mal. A. Umar; a taxonomist at the Botany unit of the Department of Biological Sciences, Usmanu Danfidiyo University, Sokoto; Nigeria. A voucher specimen number (UDUH/ANS/0066) was assigned to the sample while the specimen sample was deposited in the Herbarium of the same Department.
Formulation of the Okra- based Antidiabetic Nutraceutical:
The formulation is according to Muhammad et al [21]. Briefly, the selected okra fruits were broken to separate the seeds from the pods. The two portions (the seeds and the peels) were separately grounded to fine powdered form. The powdered samples were sieved with a fine mesh then 10 : 90 % (seed : peel) of the powdered okra seeds and peels were accurately measured, mixed thoroughly and stored in an airtight glass container at normal laboratory conditions until when required for reconstitution and administration.
Experimental Animals:
Thirty (30) apparently healthy young Wistar Albino rats of both sexes weighing between 100 - 120 g were used for this study. The rats were kept at animals house under normal environmental conditions and maintained with free access to pelletized growers feed, and water ad libitum. The animals were allowed to acclimatize for two weeks before the induction of diabetes. All procedures involving the use of animals in this research complied with the guiding principles for research involving animals as recommended by the Helsinki declaration and the guiding principles in the care and use of animals [22].
Induction of Diabetes Mellitus:
All rats, except the Normal Control Group were intra-peritoneally injected with 150 mg/kg body weight of the prepared alloxan. After 6 hours of the alloxan administration, the rats were then allowed 10 % glucose solution for the next 24 hours in other to prevent alloxan induced hypoglycemia. The animals were observed for polydipsia, polyuria, polyphagia as well as general reduction in body weight. Seventy two hours after the alloxan administration, the animals were fasted overnight and diabetes was confirmed from the rats by measuring their fasting blood glucose level with the aid of a fine test glucometer (Codex Pharma Limited). Only the rats that have fasting blood glucose level >7.0 mmol/l (126 mg/dl) were considered diabetics and included in the study [23].
Grouping of Experimental Rats and Treatments:
The rats were divided in to five (5) groups of six rats each and treated for 21 days as follows: The Normal Control (NC) and Diabetic Control (DC) groups were orally treated with normal saline (10 ml/kg) in addition to their normal diet and water; the Metformin Control (MC) group was treated with 100 mg/kg Metformin in addition to their normal diet and water while the Test groups (FX1 and FX2) were treated with 100 and 200 mg/kg body weight of the (10 : 90) Okra formulation in addition to their normal diet and water respectively.
Blood Sample Collection and Preparation of Serum:
Twenty four (24) hours after the last treatment, the animals were subjected to 12 hours fasting after which the animals were anaesthetized by dropping individual animal into a plastic jar saturated with chloroform vapor. The animal was then removes from the jar and blood samples collected from the animal through cardiac puncture. Blood was collected in labeled plastic specimen bottles containing EDTA for glycated hemoglobin assay; the remaining blood was collected in plain plastic centrifuge tube and allowed to clot then centrifuged at 4000 g for ten (10) minutes. The sera obtained from the rats were used for estimation of the serum glucose and lipid profile.
Estimation of Serum Glucose Level:
This was estimated by glucose oxidase/ peroxidase method using Randox kit [24].
Estimation of Glycated Hemoglobin (HbA1c): The Glycated Hemoglobin (HbA1c) was estimated by the method of Yazdanpanah et al., [25].
Estimation of Serum Total Cholesterol (TC):
TC was estimated by enzymatic method using Randox kit [26].
Estimation of Serum HDL- C:
This was done by enzymatic method using Randox Kit [27].
Estimation of Serum Triglyceride (TG):
This was assayed using Randox Kit[28].
Estimation of Serum LDL- C:
This was calculated using Friedewald formula [29]; LDL-C (mg/dl) = TC – (HDL - C) + ( 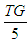 )
)
Estimation of Serum VLDL- C:
This was calculated using Friedewald formula [29]; VLDL_C (mg/dl) = 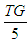
Estimation of Atherogenic Index (AI):
This was calculated as the ratio of LDL-cholesterol to HDL-cholesterol according to [30].
Data Analysis:
The data obtained were presented as mean ± standard error of the mean. Results of the Biochemical parameters were analyzed statistically by one way analysis of variance (ANOVA) followed by postHoc, Duncan multiple tests using the Statistical Package – for Social Sciences SPSS software, version 20. A p-value < 0.05 was considered statistically significant.
RESULTS
Effect of Administration of the 10: 90 Nutraceutical Formulation on Serum Glucose and Glycated Hemoglobin Levels
The results of the effect of the treatments with the FX1 and FX2 of the formulation on serum glucose and glycated hemoglobin levels were presented in Table 1. The results indicated significant (P<0.05) decrease in the level of serum glucose (5.23±0.43 mmol/l) and Glycated hemoglobin (4.78±0.17 %) in the FX1 and FX2 treated groups in comparison with that of the diabetic untreated group (14.76±1.51 mmol/l) and Glycated hemoglobin (11.04±0.31%) levels. It was also observed that, there was no significant (P > 0.05) difference in the effect of FX1 and FX2 treatments compared to Metformin.
Effect of Administration of the 10: 90 Nutraceutical formulation on Serum Lipid Profile Levels
The results of the effect of the treatments with the FX1 and FX2 of the formulation on serum lipid profile are presented in Table 2. The result indicated significant (P < 0.05) decrease in the levels of serum Total Cholesterol (TC), Triglyceride (TG), Low Density Lipoprotein (LDL-C), Very Low Density Lipoprotein (VLDL-C), and Atherogenic Index (AIX) compared with that of diabetic untreated group (DC). Also, significant (P < 0.05) increase in the level of serum High Density Lipoprotein Cholesterol (HDL-C) (48.66±4.96 mg/dl) was observed in the FX1 and FX2 treated group as compared to that of the diabetic untreated group (16.90±3.59 mg/dl). Same effect was observed in Metformin treated group.
DISCUSSION
Following the Alloxan injection, the animals displayed the expected symptoms of insulin-dependent diabetes mellitus, i.e., hyperglycemia, polydipsia, polyuria, increase in food and water intake as previously observed [1]. This could be due to the selective toxicity of alloxan on β-cells of pancreas after the alloxan injection resulting in reduced synthesis and release of insulin which leads to alteration of glucose metabolism and utilization thereby causing hyperglycemia [31]. Generally; prolonged uncontrolled high blood glucose has been shown to results in elevated levels of serum glucose, glycated hemoglobin, oxidative stress indices as well as decreased levels of antioxidants defenses and lipid abnormalities due to lipid peroxidation [7].
The significant (P < 0.05) decrease in fasting blood glucose level observed in the groups treated with FX1 and FX2 for three weeks as compared to that of diabetic untreated group (Table 1) might be attributed with the ability of the dietary fibers and the characteristic viscosity of the formulation to reduce the diffusion of glucose and delay the digestion and absorption of carbohydrates derived from the rats’ diet. This has been supported by the earlier [32]. It has also been reported that different types of dietary fibers (especially the soluble fibers) could reduce the diffusion of glucose in- vitro [32] and in- vivo [33]. Another study also [34,35] reported the hypoglycemic effect of Okra fruit might be attributed to inhibition of α- glucosidase and α- amylase enzymes.
The observed increase in the level of glycated hemoglobin (HbA1c) in the diabetic untreated group (Table 1) could be due to the persistent hyperglycemia in diabetic condition; because in diabetes, the persistent and excess amount of glucose present in the blood reacts with hemoglobin to form glycated hemoglobin which may also induce the generation of oxygen derived free radicals and other diabetes-associated complications in prolonged diabetic condition [34]. Effect of the treatment with FX1 and FX2 showed significant (P < 0.05) decrease in the glycated hemoglobin level (Table 1). Similar results were observed in the metformin treated group. The ability of the okra based nutraceutical formulation to decrease HbA1c levels in diabetic rats showed its potentials to prevent the diabetic-associated complications. This might be connected with its hypoglycemic effect as well as its antioxidants rich compounds (e.g., carotenoids, riboflavin, ascorbic acid, thiamine and nicotinic acid) identified in Okra fruit [36]. The antioxidant or free radical scavenging property in plants such as Okra fruit may inhibit oxidative reactions associated with glycation. Also, a study[16] have reported that ‘Okra have strong antioxidant properties through free radicals scavenging such as superoxide anion, hydroxyl radical and nitric oxide with strong synergic effects’.
The significant (P < 0.05) elevation in lipid profile parameters (TC, TG, LDL-C VLDL-C and AIX as well as significant (P < 0.05) decrease in the level of HDL-C ) in the diabetic untreated (DC) rats as compared to the normal control (NC) rats and that of the FX1 and FX2 treated groups of rats (Table 2) could be as a results of the facts that there is decreased secretion of insulin and increase in other hormones such as glucagon and catecholamines. These hormones are lipolytic and hence increased lipolysis resulting in the release of more free fatty acids from the peripheral deposits into the circulation [37]. The increased fatty acid concentration also increases β-oxidation of fatty acids, producing more acetyl Co-A and cholesterol hence, hypercholesterolemia and hypertriglycedemia in diabetes [38, 39]. Administration of the FX1 and FX2 of the formulation significantly (P < 0.05) reversed the diabetes induced hyperlipidemia. These results agree with previous reports on the antilipidemic properties of Okra fruits [40,41,42]. This may be due to the attainment of normoglycemia as a result of the hypoglycemic effects of the FX1 and FX2 administration. Also, the presence of some macro and micro nutrients as well as other vital antioxidant substances in okra fruit [36] may work in a way similar to the effect of insulin or enhance insulin sensitivity / secretion of insulin from the beta cells of pancreas. This may leads to increase in uptake of glucose and thereby decrease the rate of lipolysis. Further, the hypolipidemic effect of the formulation may also be associated with the fiber / mucilage content of the formulation which could decrease the absorption of dietary cholesterol from the intestine. It has been reported that Okra fruit is rich in pectin in addition to other dietary fiber content [43]. Pectin helps in reducing high blood cholesterol by modifying the synthesis of bile within the intestines. [43]. It could also binds with the bile salts and reduces their enterohepatic circulation there by resulting in increased degradation of cholesterol to bile salts [43]. This corroborated the findings of [40] which reported that “the Hypolipidemic Activity of Okra is mediated through inhibition of lipogenesis and upregulation of cholesterol degradation” [40].
Conclusion
Based on these findings, the nutraceutical formulation (10:90) peel and seeds of Ex-maradi okra fruit possessed significant hypoglycemic and hypolipidemic activity in alloxan-induced diabetic rats and is suitable for the development of Okra–based nutraceutical for management of diabetes mellitus.
References:
-
Muhammad I, Matazu KI, Yar’adua AI, Nasir A, Matazu NU, Zainab AS, Abdul Rahman MB, Bilbis LS, Abbas AY. Antidiabetic Activity of Abelmoschus esculentus (Ex- Maradi Okra) Fruit in Alloxan-induced Diabetic Rats. Nigerian Journal of Biochemistry and Molecular Biology. 2017; 32(1): 44-52.
-
Ilias M, El-Mostafa K, Nezha S, Bouchra M, Mourad K, Azlarab M, et al; In Vitro and In Vivo Antioxidant and Anti-Hyperglycemic Activities of Moroccan Oat Cultivars. Antioxidants. 2018; 6 (102): 1-20.
-
Kaur CD, Talreja S. Fighting diabetes with herbal technological developments. Review: world journal of pharmaceutical research. 2014; 3(2): 2842-2862.
-
Jamal G, Mohammed JG, Mohammed B. Supplementation with Rich-Polyphenols olive Tree Powder Improves Fasting Blood Glucose and Insulin Resistance in Patients with Type 2 Diabetes Mellitus: A 14-Weeks Randomized, Double-Blind, Placebo-Controlled Clinical Trial. International Journal of Science and Research (IJSR). 2018; 7(5): 545- 551.
-
Michael PK, Asim AB, Robert SB. The Utility of Oral Diabetes Medications in Type 2 Diabetes of the Young. Current Diabetes Review. 2005; 1: 83-92.
-
Niu CS, Chen W, Wu HT, Cheng KC, Wen YJ, Lin KC, Cheng JT. Decrease of plasma glucose by allantoin, an active principle of yam (Dioscorea spp.), in streptozotocin-induced diabetic rats. Journal of Agric and Food Chemistry. 2010; 58, 12031–12035.
-
Hyeon-Kyu GM, Mahbubur-Rahman M, Gi-Beum K, Chong-Sam N. et al; Antidiabetic Effects of Yam (Dioscorea batatas) and Its Active Constituent, Allantoin, in a Rat Model of Streptozotocin-Induced Diabetes. Nutrients. 2015; 7, 8532–8544.
-
World Health Organization (WHO) WHO Study Group Report on Prevalence of Diabetes Mellitus. (WHO Technical Report Series. 2011; No: 876).
-
Rao MU, Sreenivasulu M, Chengaiah B. et al; Herbal Medicines for Diabetes Mellitus: A Review. International Journal of PharmTech Research. 2010; 2 (3): 1883- 1892.
-
Kaushik K, Sharma AK, Agarwal V. Formulation and Evaluation of Herbal Antidiabetic Tablet. Journal of Drug Delivery & Therapeutics. 2011; 1(1): 65-67.
-
Chandira M, Jayakar V. Formulation and Evaluation of Herbal Tablets Containing Ipomoea Digitata Linn. Extract. International Journal of Pharmeutical Science Research. 2010; 3(1): 101- 110.
-
Hasan I, Khatoon S. Effect of Momordica Charantia (Bitter gourd) Tablets in Diabetes Mellitus: Type 1 and Type 2. Prime Research on Medicine. 2012; 2(2): 72-74.
-
Pari L, Ramakrishnan R, Venkateswaran S. Antihyperglycaemic Effect of Diamed, a Herbal Formulation, in Experimental Diabetes in Rats. Journal of Pharmacy and Pharmacology. 2001; 53 (8): 1139-1143.
-
Agrawal P, Rai V, Singh RB. Randomized placebo controlled single blind trial of holy basil leaves in patients with noninsulin-dependent diabetes mellitus. International Journal of Clinical Pharmacological Therapy. 1996; 34: 406-409.
-
Hui H, Tang G, Liang VW. Hypoglycemic Herbs and their Action Mechanisms. Journal of Chinese Medicine. 2009; 4: 1-11.
-
Ortaç D, Mustafa C, Turan K, Mehmet E, Büyükokuro?lu ZÖ, Özdemir ATK, Sad?k G, In vivo anti-ulcerogenic effect of okra (Abelmoschus esculentus) on ethanol-induced acute gastric mucosal lesions. Pharmacological Biology. 2018; 56(1): 165–175.
-
John RT, Perez RJ, Baritua MO, Pacalna SO Malayao J. Exploratory Investigation on the Hypoglycemic Effect of Abelmoschus Esculentus in Mice. International Journal of Scientific & Technology Research. 2013; 1 (2): 249.
-
Indah MA. Differential Expression Analysis of Diabetic Related Genes in Streptozotocin Induced Diabetic Rats in Response to Abelmoschus esculentus Treatment: A Research Framework. International Conference on Bioscience, Biochemistr & Bioinformatic. Singapore. 2011; 1: 5.
-
Sathish KD, Srinivasa RB, Durga PG, Brahma SR, Nadendla R. Vijayapandi P, Shankul K. Physicochemical, Phytochemical and Toxicity Studies on Gum and Mucilage from Plant (Abelmoschus esculentus). The Journal of Phytopharmacology. 2014; 3(3): 200-203.
-
Subrahmanyam GV, Sushma M, Alekya A, Neeraja H, Sai Sri H, Ravindra J. Antidiabetic Activity of Abelmoschus esculentus Fruit Extract. International Journal of Research in Pharmaceutical Chemistry. 2011; 1 (1): 231.
-
Muhammad I, Matazu KI, Yaradua IA, Yau S, Nasir A, Bilbis LS, Abbas AY. Development of Okra-Based Antidiabetic Nutraceutical Formulation from Abelmoschus esculentus (L.) Moench (Ex-maradi Variety). Tropical Journal of Natural Product Research. 2018; 2(2): 80-86.
-
World Medical Association. American Physiological Society. Guiding principles for research involving animals and human beings. Am. J. physiol. Reg. Comp. Physiol. 2002; 283: 281-281.
-
Kaliwal BB, Shetti AA, Sanakal RD, Antidiabetic effect of ethanolic leaf extract of Phyllanthus amarus in alloxan induced diabetic mice. Asian Journal of Plant Science and Research. 2012; 2 (1): 11-15.
-
Trinder P. Determination of Blood Glucose in Blood Using Glucose Oxidase with an Alternative Oxygen Acceptor. Annals Clin. Biochemistry. 1969; 6: 24-25.
-
Yazdanpanah S, Rabiee M, Tahriri M, Abdolrahim M, Tayebi L. Glycated hemoglobin-detection methods based on electrochemical biosensors. TrAC Trends Anal. Chem. 2015; 72, 53–67.
-
Allain CC, Poon LS, Chan CSG, Richmond W, Fu PC. Enzymatic Determination of Total Serum Cholesterol. Journal of Clinical Chemistry. 1974; 20: 470.
-
Burstein M, Scholnick HR, Morfin R. Rapid Method for the Isolation of Lipoproteins from Human Serum by Precipitation with Polyanions. Joural of Lipid Research. 1970; 11: 583-595.
-
Tietz NW. Serum Triglyceride Determination. In: Clinical Guide to Laboratory Tests. Second Edition, W.B., Saunders Co., Philadelphia, USA. 1990; 554-556.
-
Friedewald WT, Levy RI, Fredrickson DS. Estimation of LDL-C in Plasma without the use of the Preparative Ultracentrifuge. Clinical Chemistry. 1972; 18 (6): 499-502.
-
Abbott RD, Wilson PW, Kannel WB, Castelli WP. High Density Lipoprotein Cholesterol, Total Cholesterol Screening and Myocardial Infarction. The Framingham Study. Atheros., 1988; 8: 207-211.
-
Arumugam S, Kavimanib S, Kadalmanic B. Antidiabetic Activity of Leaf and Callus Extracts of Aegle Marmelos in Rabbit. Sci. Asia. 2008; 34: 317-321.
-
Ou S, Kwok K, Li Y, Fu L. In-vitro study of possible role of dietary fiber in Lowering Postprandial Serum Glucose. Journal of Agriculture and Food Chemistry. 2001; 49: 1026-1029.
-
Lafrance L, Raabasa-Lhore, R, Poisson D, Ducros F, Chiasson JL. Effects of Different Glycemic Index Foods and Dietary fiber Intake on Glycemic Control in Type I Diabetic Patients on Intensive Insulin Therapy, Diabetes Med. 1998; 15: 972-978.
-
Alyassin, D, Ibrahim K. A Minor Haemoglobin Fraction and the Level of Fasting Blood Glucose. Jounal of Faculty of Medicine. University of Baghdad. 1981; 23: 373- 380.
-
Chanida P, Sanya H, Tanasorn T, Nijsiri R. In vitro glucose entrapment and alphaglucosidase inhibition of mucilaginous substances from selected Thai medicinal plants. Scientia Pharmaceutica 2009; 77:837-849.
-
Habtamu FG, Negussie R, Gulelat DH, Ashagrie Z. Nutritional Quality and Health Benefits of Okra (Abelmoschus esculentus): A Review, Global Journal of Medical Research. 2014; 14 (5): 1.
-
Agardh CD, Bjorgell P, Nilson EP. The Effect of Tolbutamide on Lipoproteins and Lipoproteinlipase and Hormone Sensitive Lipase. Diabetes Research Clinical Practice. 1999; 46: 99-108.
-
Mironova MA, Klein RL, Virella GL, Lopes-Virella MF. Anti-modified LDL Antibodies, LD -containing Immune Complexes and Susceptibility of LDL to in -vitro Oxidation in Patients with Type 2 Diabetes. Diabetes. 2000; 49: 1033-1049.
-
Howard BV, Robbins DC, Sievers ML. et al., “LDL Cholesterol as a Strong Predictor of Coronary Heart Disease in Diabetic Individuals with Insulin Resistance and Low LDL: the Strong Heart Study.” Arteriosclerosis, Thrombosis, and Vascular Biology. 2000; 20 (3): 830–835, 2000.
-
Hong W, Gu C, Dandan R, Shang-taian Y. Hypolipidemic Activity of Okra is Mediated Through Inhibition of Lipogenesis and Upregulation of Cholesterol Degradation Phytotherapy Research. 2013; 28(2): 268-273.
-
Poorva Dubey and Sunita Mishra. A review on: Diabetes and okra (Abelmoschus esculentus) Journal of Medicinal Plants Studies. 2017; 5(3): 23-26.
-
Suraksha M. A Review on Okra as an Antidiabetic, Antioxidant and An Excellent Energy Source. Organic and Medicinal Chemistry International Journal. 2018; 6 (1): 1-5.
-
Viuda-Martos M, Opez-Marcos MCL, Fern´andez- L´opez J, Sendra E, L´opez-Vargas, JH, P´ erez-´Alvarez JA. Role of Fiber in Cardiovascular Diseases: Comprehensive Review on Food Science and Food Safety. 2010; 9: 240-258.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License