IJCRR - 10(7), April, 2018
Pages: 06-11
Date of Publication: 14-Apr-2018
Print Article
Download XML Download PDF
Rationale for Palliative Gastrectomy and Metastasectomy in Metastatic Gastric Cancer
Author: Abdullah Sakin, Makbule Tambas, Nurgul Yasar, Caglayan Geredeli, Saban Secmeler, Cumhur Demir, Ali Alemdar, Hakan Guven, Sener Cihan
Category: Healthcare
Abstract:Objectives: Approximately 50% of gastric cancer patients are locally advanced or metastatic staged at the time of diagnosis. In limited metastatic patients, performing surgery is thought to be related to survival benefit. Thus, we evaluated the effect of the surgery on survival in patients with metastatic gastric carcinoma who have been treated and followed-up in our oncology clinic.
Methods: Patients with pathologically verified metastatic gastric cancer between 2009-2016 were included in the study.The patients were divided into 3 groups as those who underwent palliative gastrectomy (group A), who underwent simultaneous gastric surgery and metastasectomy (group B), and who underwent no surgery (group C).
Results: One hundred and fifty-three patients, including 35 in the group A, 10 in the group B, and 108 in the group C, were included in the study. There was a significant difference between the groups in terms of the mean age of the patients (60,4, 53 and 63, respectively; p=0.016). Median follow-up time was 8\?9.6 months. Medianoverall survival (OS) was 20 months in group
B, 13 months in group A and 6 months in group C;while OS was found to be significantly increased in surgery groups compared with non-surgery (Group A vs. B, p=0,259; group A vs. C, p< 0,001; group B vs. C, p< 0,001).
Conclusions: It is determined that performing surgery for primary tumor and/or its oligomatastases provided survival benefit
in patients with good performance in metastatic gastric cancer. Further prospective studies with higher number of patients are
warranted in this subject.
Keywords: Chemotherapy, Gastrectomy, Gastric cancer, Metastasectomy
DOI: 10.7324/IJCRR.2018.1072
Full Text:
INTRODUCTION
Gastric cancer is the fifth most common cancer around the world and is the third most common cause of cancer-related deaths. In 2017, 28,000 new gastric cancer cases and 10,960 gastric cancer-related deaths are expected in the United States(1). At the time of diagnosis, approximately 50% of patients are locally advanced or metastatic while nearly half of non-metastatic patients are eligible for curative surgical treatment(2). Moderate improvements in the gastric cancer prognosis in recent years may adhere to improvements in multidisciplinary treatment modalities, such as improved surgical technique and the use of new chemotherapy regimens(1, 2).
Surgical resection of the primary tumor in patients with locally advanced or metastatic disease may provide palliation of symptoms such as nausea, pain, obstruction or bleeding. The results of some studies suggest that palliative gastrectomy may be associated with survival benefit in patients with oligometastatic disease. This benefit, however, has not been seen in all studies (2, 3).
We investigated whether surgery provided any survival advantages in patients who were treated and followed-up in our oncology clinic for metastatic gastric carcinoma and underwent palliative gastrectomy, simultaneous gastrectomy and metastasectomy and no surgical intervention.
MATERIALS AND METHODS
Patients with pathologically verified metastatic gastric cancer between 2009-2016 were included in the study. The clinical characteristics data of patients including age, sex, smoking, comorbidities, type of surgery performed, pathological features, sites of metastases, ECOG performance status, treatment details were obtained retrospectively from patients' medical file. Patients whose data were not accessable and who werw diagnosed with multiple cancer were excluded from the study. The patients were divided into 3 groups as those who underwent palliative gastric surgery at the time of diagnosis (Group A), who underwent simultaneous gastric surgery and metastasectomy at the time of diagnosis (Group B) and who underwent no surgery (Group C). Overall survival (OS) was calculated from the date of diagnosis to the date of death or last contact with patient.
Statistical Analysis
SPSS 22.0 for Windows program was used for statistical analysis. Descriptive statistics were given as mean, standard deviation, minimum, maximum for numerical variables, number and percentage for categorical variables. The numerical variables in the independent two groups were analyzed by Student t test and Mann Whitney U test if normal distribution condition was provided and not met, respectively. The comparisons of ratios between groups were made with Chi Square Analysis. Monte Carlo simulation was applied when conditions were not met. The survival analyzes were performed with Kaplan Meier Analysis. Determinants for survival were examined by Cox Regression Analysis. Statistical significance level of alpha was accepted as p <0,05.
RESULTS
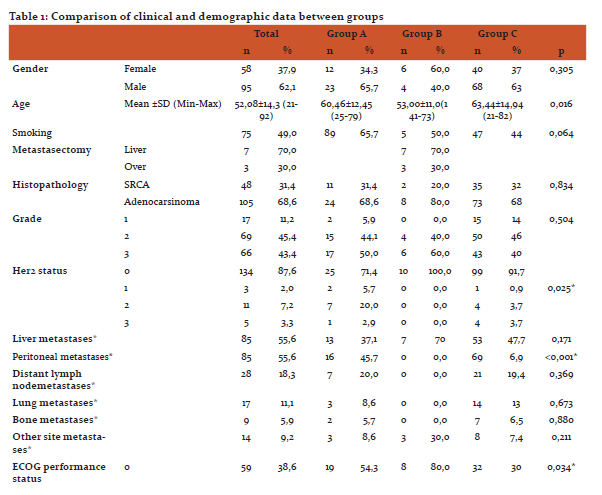
One hundred and fifty-three patients, 35 in group A, 10 in group B and 108 in group C, were included in the study. Of the patients, 95(62,1%) were male and 58(37,9%) were female while there was no difference in terms of gender between the groups (p = 0,305). A significant difference in the mean age of the patients was detected between the groups (60,4, 53 and 63, respectively; p<0,016). Similarly, performance scores (ECOG PS) differed significantly between the groups (p 0.034). In Group B, the ECOG PS 0 ratio was 80%. There was no difference in smoking among the groups (0,064).
There was no statistically significant difference between the groups in terms of histopathology (p0,834) and grade (p=0,504). In Group B, 7 patients had liver metastasectomy and 3 patients had over metastasectomy. At diagnosis, peritoneal metastasis was significantly higher in group C compared with others (p =0.001)whereas incidence of other metastatic sites at dignosis were found to be similar between groups. There was no difference in the rates of the first, second and third lines of chemotherapy treatment administration among patients.
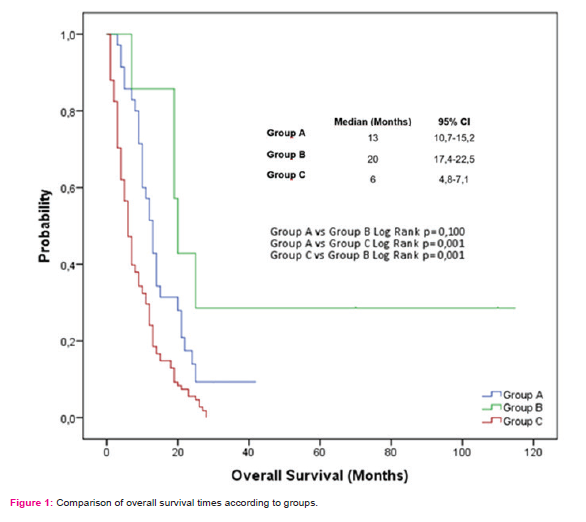
Mean follow-up time was 13±8,2 months in group A, 19,5±25,9 months in group B and 6±7,1 months in group C. During the follow-up, 85.7% of the patients in Group A, 50% of the patients in Group B and all of the patients in Group C died. Clinical and demographic data of the patients are summarized in Table 1. OS was observed as 13 months in group A, 20 months in group B and 6 months in group C (Figure 1).
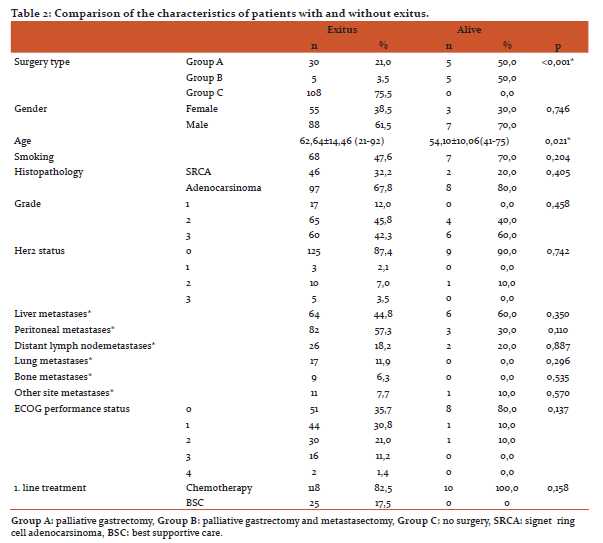
Surgical type (p<0.001) and the age of patient at diagnosis (p=0.021) were found to be significant factors in the univariate analysis for the OS(Table 2).When factors affecting the OS were evaluated, performing surgery was found to be the most significant factor according to Forward Stepwise analysis based on model consisted of variables of which p values were determined as <0,250 in univariate analysis (type of surgery, age, smoking, the presence of peritoneal metastases, first line treatment) (p <0.001). Furthermore, the median OS was found to be significantly increased in surgery groups compared with non-surgery according to the Kaplan Meier analysis (Group A vs. B, p=0,259; group A vs. C, p<0,001; group B vs. C, p=<0,001) (Figure 1).
DISCUSSION
In recent years, surgical treatment in metastatic gastric cancer remains controversial. Although chemotherapy is the standard treatment, long-term survival has recently been reported particularly with surgical treatment in selected patients with metastatic liver cancer(4, 5). However, most patients are locally advanced or metastatic at the time of diagnosis. The average survival of these patients is approximately 12 months (6). Similarly, in our study, the median survival time of group-independent patientswas 11.5 months.
Liver metastases in gastric cancer are rarely found suitable for surgery. Generally, the disease is presented with multiple liver metastases involving both lobes. Long-term survival after resection of isolated liver metastases is extremely rare. To our knowledge, there is no prospective study on liver metastasectomy in gastric cancer. Instead, there are mostly retrospective studies with few cases(7, 8).
In a retrospective study of 17 patients with gastric cancer with liver metastasis, the 5-year survival rate was 31.5%, the mean survival time was 34 months after liver metastasectomy(9). In another study with 15 gastric cancer patients who had synchronous or metachronous isolated liver metastasis, the patient underwent liver resectionif the metastatic lesions wereaccepted as completely resectable during surgery or radiofrequency ablation. The OS was 48 months in patients who received curative treatment for liver metastasis compared with 9 months who did not(10). In our study, 7 patients underwent synchronous liver metastasectomy and the OS of these patients were 19 months.
Over metastasis is usually seen as synchronously or metachronously in female patients. Over metastasis in gastric cancer is associated with poor prognosis. The role of over metastasectomy is still under debate and will probably beneficial in a particular group of patients(11). In a retrospective study of 85 patients with over-metastasized gastric cancer, 35 patients underwent metastasectomy, and longer OS was obtained in patients undergoing metastasectomy (14.1 months)(12). Recently, in a retrospective study including 93 patients with synchronous over metastases, the OS of patients who underwent over metastasectomy (n=49) were significantly better than those who did not (n=44) (19.0 vs 11.8 months, p<0.001)(13). In our study, synchronous over metastasectomy was quite rare (n=3) and the OS of these patients were 20 months.
In a retrospective study of 333 patients with metastatic gastric cancer by Hsu et al., 133 patients underwent palliative gastrectomy and a significantly longer median OS was detected (7.7 vs. 4.9 months)(14). Similarly, in the study by Kim et al. which had identical design with our study, 274 patients with metastatic gastric cancer who received chemotherapy were divided into 3 groups; primary tumor and metastatic lesions resection, palliative gastrectomy and only chemotherapy. The mean OS was 28, 15, 5, and 9 months respectively, and 3-year survival rates were 42, 8, 8, 1, and 3, 5%, respectivelyand their results are convenient with ours (15).In our study, a portion of patients who did not undergo surgery (18%) received only best supportive care. A significant OS benefit was achieved in patients who underwent surgical treatment compared with who did not.
The most important deficiency of the studies is ignorance of the factors such as low disease burden, good performance status, and the use of postoperative systemic chemotherapy in patients who are candidates for surgical resection. Likewise, it has been seen that the performance of the patients who underwent surgery was better, and also some of the patients who did not undergo surgery received only best supportive care since they were not eligible for chemotherapy in our study.
Before deciding on operation at the metastatic stage, the patient's accompanying comorbidities, performance status, estimated duration of survival, and severity of symptoms should be considered. In general, it is unlikely that all patients with liver metastasized gastric cancer will benefit from metastasectomy. However, it may be an option for carefully selected patients with limited metastasis. Although the role of over metastasectomy is still debated, it is likely to contribute to survival in a particular group of patients. Resection for palliation usually results in symptomatic relief and longer survival in appropriately selected patients. In daily practise, palliative gastrectomy should be used in selected patients for symptomatic palliation in cases for whom other treatment options are not approtiate.
CONCLUSION
As a result, there is no consensus on the criteria for selection of surgical treatment candidates in metastatic gastric cancer. Our study results suggest that the surgical treatment of the primary tumor and/or its oligomatastases in appropriate metastatic gastric cancer patients provided survival benefit. Further prospective studies with higher number of patients are warranted in this subject to make a more robust conclusion.
ACKNOWLEDGEMENT
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
ETHICAL CLEARANCE
The study was approved by the Institutional ethics board and found to comply with ethical principles for epidemiological investigations.
SOURCE OF FUNDING:None
CONFLICT OF INTEREST: The authors declare that they have no conflict of interest.
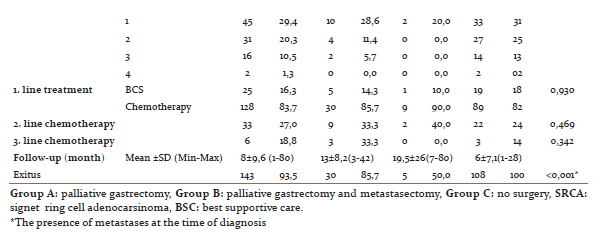

References:
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30.
2. Jeong O, Park YK, Choi WY, Ryu SY. Prognostic significance of non-curative gastrectomy for incurable gastric carcinoma. Ann Surg Oncol. 2014;21(8):2587-93.
3. Chen J, Kong Y, Weng S, Dong C, Zhu L, Yang Z, et al. Outcomes of surgery for gastric cancer with distant metastases: a retrospective study from the SEER database. Oncotarget. 2017;8(3):4342-51.
4. Kodera Y, Fujitani K, Fukushima N, Ito S, Muro K, Ohashi N, et al. Surgical resection of hepatic metastasis from gastric cancer: a review and new recommendation in the Japanese gastric cancer treatment guidelines. Gastric Cancer. 2014;17(2):206-12.
5. Martella L, Bertozzi S, Londero AP, Steffan A, De Paoli P, Bertola G. Surgery for Liver Metastases From Gastric Cancer: A Meta-Analysis of Observational Studies. Medicine (Baltimore). 2015;94(31):e1113.
6. Tegels JJ, De Maat MF, Hulsewe KW, Hoofwijk AG, Stoot JH. Improving the outcomes in gastric cancer surgery. World J Gastroenterol. 2014;20(38):13692-704.
7. Linhares E, Monteiro M, Kesley R, Santos CE, Filho OS, Simoes JH. Major hepatectomy for isolated metastases from gastric adenocarcinoma. HPB (Oxford). 2003;5(4):235-7.
8. Cheon SH, Rha SY, Jeung HC, Im CK, Kim SH, Kim HR, et al. Survival benefit of combined curative resection of the stomach (D2 resection) and liver in gastric cancer patients with liver metastases. Ann Oncol. 2008;19(6):1146-53.
9. Tsujimoto H, Ichikura T, Ono S, Sugasawa H, Hiraki S, Sakamoto N, et al. Outcomes for patients following hepatic resection of metastatic tumors from gastric cancer. Hepatol Int. 2010;4(1):406-13.
10. Dittmar Y, Altendorf-Hofmann A, Rauchfuss F, Gotz M, Scheuerlein H, Jandt K, et al. Resection of liver metastases is beneficial in patients with gastric cancer: report on 15 cases and review of literature. Gastric Cancer. 2012;15(2):131-6.
11. Rosa F, Marrelli D, Morgagni P, Cipollari C, Vittimberga G, Framarini M, et al. Krukenberg Tumors of Gastric Origin: The Rationale of Surgical Resection and Perioperative Treatments in a Multicenter Western Experience. World J Surg. 2016;40(4):921-8.
12. Lu LC, Shao YY, Hsu CH, Hsu C, Cheng WF, Lin YL, et al. Metastasectomy of Krukenberg tumors may be associated with survival benefits in patients with metastatic gastric cancer. Anticancer Res. 2012;32(8):3397-401.
13. Yu P, Huang L, Cheng G, Yang L, Dai G, Ying J, et al. Treatment strategy and prognostic factors for Krukenberg tumors of gastric origin: report of a 10-year single-center experience from China. Oncotarget. 2017;8(47):82558-70.
14. Hsu JT, Liao JA, Chuang HC, Chen TD, Chen TH, Kuo CJ, et al. Palliative gastrectomy is beneficial in selected cases of metastatic gastric cancer. BMC Palliat Care. 2017;16(1):19.
15. Kim KH, Lee KW, Baek SK, Chang HJ, Kim YJ, Park DJ, et al. Survival benefit of gastrectomy +/- metastasectomy in patients with metastatic gastric cancer receiving chemotherapy. Gastric Cancer. 2011;14(2):130-8.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License