IJCRR - 10(6), March, 2018
Pages: 37-42
Date of Publication: 28-Mar-2018
Print Article
Download XML Download PDF
Effect of Duration of Diabetes on Heart Rate Variability in Type 2 Diabetes Mellitus
Author: Amina Sultan Zaidi, P. N. Singh, Meenakshi Gupta, Sheelu Shafiq Siddiqi
Category: Healthcare
Abstract:Introduction: Diabetes is a metabolic disorder with debilitating complications which affects several million people worldwide. Cardiac Autonomic Neuropathy (CAN) is the most common chronic complication of type 2 diabetes mellitus (T2DM), which is concealed for long period of disease. Heart rate variability (HRV) test is the simple and sensitive test for CAN. Diagnosing CAN at subclinical stage and knowing the correlation of HRV with duration of diabetes can help to prevent the morbidity and mortality because of this neuropathy.
Objective: This study aims to determine the correlation of HRV with duration of diabetes in type 2 diabetes mellitus.
Materials and Methods: This cross sectional study was conducted in Department of Physiology, in collaboration with Rajeev Gandhi Centre for Diabetes and Endocrinology on patients of T2DM attending Diabetes clinic in Jawaharlal Nehru Medical college hospital (JNMCH), AMU, Aligarh, from 2014 to 2016 after approval from the ethical committee of J. N. Medical College. Total 90 subjects were taken, among whom 60 subjects were diagnosed cases of T2DM, which were divided in two groups on the basis of duration of diabetes. Rest 30 subjects were non diabetic control group. For CAN heart rate variability test was done.
Statistical analysis was performed using SPSS version 21.0.
Results: In shorter duration Diabetes (3.31 \? 1.72 years T2DM), significant (p value< 0.05) negative correlation was seen between duration of diabetes and pNN50, RMSSD, HF power and LF/HF ratio, while in longer duration of Diabetes (12 \? 4.42 years T2DM), there was significant (p value< 0.05) negative correlation between duration of diabetes and all four HRV parameters, namely, pNN50, RMSSD, HF power, LF power, LF/HF ratio.
Conclusion: Chronic hyperglycemia in diabetes leads to the degeneration and damage of autonomic nerves.
Keywords: Cardiac Autonomic Neuropathy, Chronic hyperglycemia, Low frequency power, High frequency power
DOI: 10.7324/IJCRR.2018.1068
Full Text:
INTRODUCTION:
Diabetes Mellitus (DM) is a metabolic disease characterized by hyperglycemia which results from defects in insulin secretion, insulin action or both. [1]. Diabetes is a debilitating disease which affects several million people worldwide. With a national DM prevalence of 8.6% and 668,468,800 numbers of people with DM, India stands second to China in relation to the burden of DM [2].
Patient with diabetes are at much higher risk for development of both microvascular and macrovascular complications, including peripheral neuropathy, nephropathy, retinopathy, and cardiovascular disease [3].
Autonomic nerve damage can be found in many diabetics often without accompanying symptoms. Cardiac autonomic neuropathy (CAN) is observed to be the most commonly seen diabetic complications and yet it is the most ignored one as well. CAN is now generally recognized as an independent risk factor for cardio-vascular diseases [4], [5]. The high mortality rate seen in diabetic patients is largely attributable to cardiac arrhythmias, silent myocardial ischemia, perioperative cardiovascular, and cardiorespiratory instability [6]. So, early diagnosis of cardiac autonomic neuropathy is required to prevent the debilitating complications. Heart rate variability test is one of the significant sensitive and early predictor of cardiac autonomic neuropathy, and which can be used for early deduction of complications among diabetes mellitus patients. It has been suggested that CAN diagnosis may be used for cardiovascular risk stratification in patients with and without established cardiovascular disease, as a marker for patients requiring more intensive pharmacotherapeutic and life-style management of co morbid conditions [7].
So this study was undertaken to study the effect of duration of diabetes on heart rate variability parameters in type 2 diabetes mellitus patients.
MATERIAL AND METHOD:
The present study was conducted in Rajeev Gandhi Centre for Diabetes and Endocrinology and Department of Physiology on patients of Type 2 Diabetes Mellitus (T2DM) attending Diabetes clinic in Jawaharlal Nehru Medical College & Hospital, AMU, Aligarh, from November 2014 to May 2016 after approval from the ethical committee of J. N. Medical College. Design of the study was cross sectional and total 90 subjects were taken. Out of these 90 subjects 60 were selected as cases for further study who met the inclusion and exclusion criteria and gave the valid consent in writing after explaining the procedure to the subject prior to entering for further investigations. A detailed history and physical examination was carried out for every subject who entered the study as per a pre-designed proforma. Selected cases of T2DM patients between age 30 to 69 years were assessed for diabetic cardiac autonomic neuropathy through heart rate variability test and were asked to report endocrinology laboratory after an overnight fasting of 10-12 hours in fasting state. Blood samples were collected in EDTA-Na vials for estimation of HbA1C Fluoride vials for plasma glucose, in plain vials for serum lipids and lipoproteins and serum creatinine. Blood for fasting and post prandial glucose estimation were collected on the same day.
All diabetic patients were divided in two groups based on duration of T2DM.
Group A1 (n=30) with less than 8 years
Group A2 (n= 30) with equal to or more than 8 years.
The findings were also compared with group of 30age, sex and BMI matched control group (Group A) who were free from diabetes mellitus, hypertension, coronary artery disease or any other illness which could hamper with the test results. The inclusion criteria of T2DM patients for this study were patients aged 30 - 69 years diagnosed with Diabetes on the basis of revised American Diabetic Association Criteria i.e. fasting plasma glucose 126 mg/dl (≥6.1 mmol/1) and 2 hours postprandial plasma glucose ≥ 200 mg/dl ( ≥1 1.1 mmol/1) [8]. The exclusion criteria of these patients were previous history of cardiac arrhythmia, hypertension. heart block, clinical coronary artery disease, and presence of thyroid disease (hypo or hyperthyroidism).
Heart rate variability was analysed with a PHYSIO-PAC SOFTWARE SYSTEM (Medicaid systems, Chandigarh 160002, India).Two –lead electrocardiographic data were recorded for 5 minutes and were downloaded to the HRV software analyser. Time domain analysis was done by Fast Fourier transformation. In the time domain we measured the root mean square of successive R-R interval differences (RMSSD) and the percentage of beats with a consecutive R-R interval difference >50ms (pNN50). In the frequency domain we measured high frequency power (HF), low frequency power (LF) and the LF/HF ratio.
STATISTICAL ANALYSIS
Analysis was performed using SPSS version 21.0 statistical package for windows (SPSS, Chicago, IL). Continuous variables were expressed as mean ± Standard Deviation (S.D) or range, and qualitative data was expressed in percentages. One way ANOVA with Post Hoc Tukey test were used for comparison of means between three groups. The association between continuous variables was tested by linear correlation using Pearson’s coefficient. All tests were two tailed, confidence intervals were calculated at 95% level and a p-value of < 0.05 was considered significant.
RESULT AND OBSERVATIONS:
In this study, a total of 60 patients with diagnosed type 2 diabetes mellitus were covered, who met the inclusion and exclusion criteria and had given valid consent in writing. These 60 patients were selected in a manner to segregate them in two groups of 30 each. One group of thirty patients had T2DM of less than 8 years duration with mean duration of 3.31 ±1.72 years, whereas the second group of thirty patients had T2DM for more than 8 years duration with mean duration of 12.80 ± 4.42 years. A group of 30 normal individuals without diabetes served as control in this study. The observations made during the study are as follow.
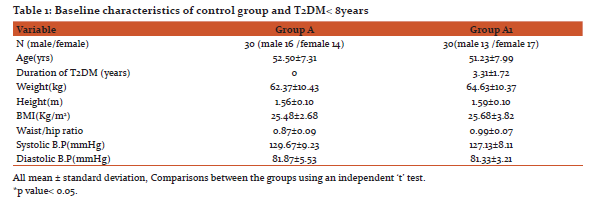
Baseline characteristics of Non-Diabetic control group, patients with more than 8 years Type 2 Diabetes mellitus and patients with less than 8 years Type 2 Diabetes mellitus are given in Table1and Table 2 respectively.
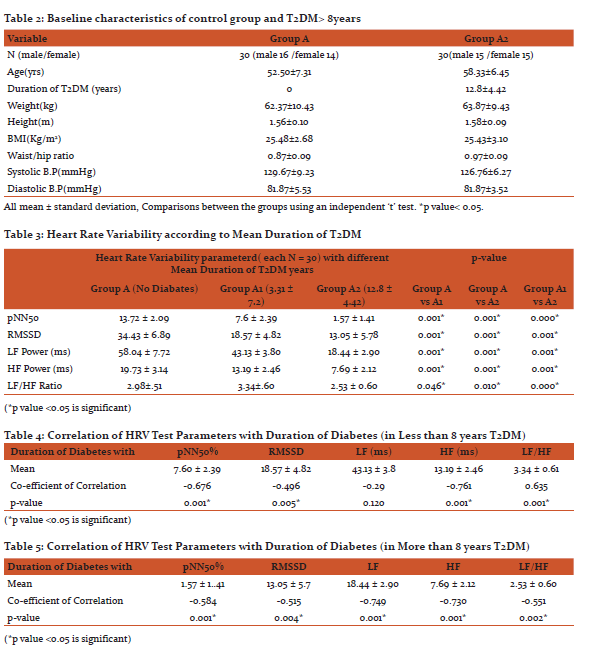
To assess Cardiac Autonomic Neuropathy Heart Rate Variability test was done. The heart rate variability parameters of the diabetes patients and control subjects are given in Table 3.
Variation in means of Heart rate Variability test parameters between non diabetes and T2DM less than 8years show significant decrease in pNN50%, RMSSD, LF power, HF power and LF/HF ratio. Variation in means of Heart rate Variability test parameters between non diabetes and T2DM more than 8years also show significant decrease in pNN50, RMSSD, LF power, HF power, and LF/HF ratio. Variation in means of Heart rate Variability test parameters between T2DM less than 8years and T2DM more than 8years show significant decrease all HRV parameters pNN50%, RMSSD, LF power, HF power, and LF/HF ratio as shown in Table 3.
Significant negative correlation is seen between duration of diabetes in T2DM of less than 8years and these HRV parameters, namely pNN50%, RMSSD, HF and LF/HF ratio. There is non-significant correlation between HRV parameter LF power and duration of diabetes as shown in Table 4.
Significant negative correlation is seen between duration of diabetes in T2DM of more than 8years and these HRV parameters, namely, pNN50%, RMSSD, LF power, HF power and LF/HF ratio (Table 5).
DISCUSSION:
Type 2 Diabetes Mellitus is a widely prevalent disease characterized by insulin insensitivity, which occurs as a result of insulin resistance and declining insulin production, eventually leading to pancreatic beta cell failure [9],[10]. As a result of this dysfunction, hyperglycemia occurs.
This study was designed to see presence of Cardiac autonomic Neuropathy through heart rate variability test, its correlation with duration of diabetes and glycemic control, in Type2 Diabetes Mellitus patients. Baseline characteristic of type 2 diabetic patients and age, sex and BMI matched healthy controls were compared. Type 2 Diabetes Mellitus patients were included in study and divided in two groups based on duration of disease.
The statistics compiled as part of this study show that duration of T2DM had considerable impact on cardiac autonomic function when assessed through heart rate variability test. Time domain and frequency domain parameters were chosen as a method for characterizing HRV in accordance with the recommendations of Task Force of the European Society of Cardiology[11]and work done in these studies[12], [13]. These recommendations suggest that these parameters are sensitive measure of HRV. The time domain parameters observed were pNN50 and RMSSD, whereas the frequency domain parameters studied were LF power, HF power and LF/HF ratio.
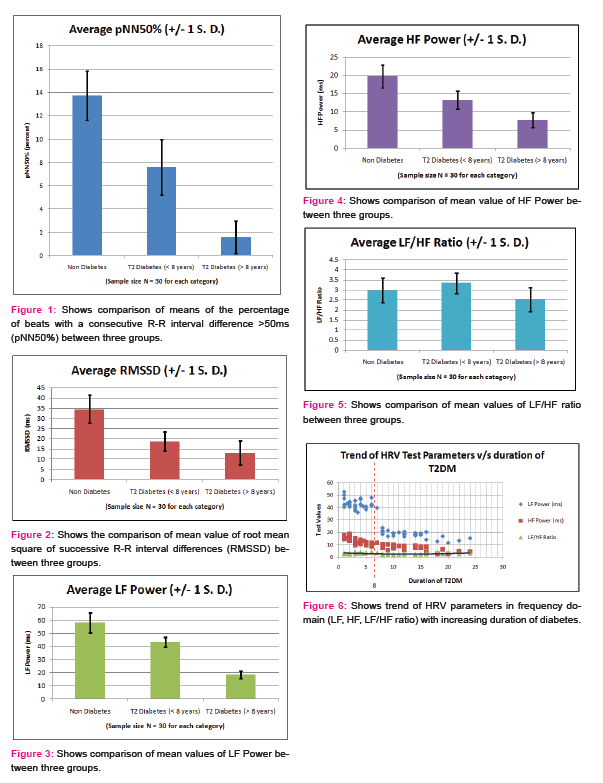
- High frequency power spectrum of HRV test is conventionally suggestive of cardiac parasympathetic activity whereas low frequency spectrum usually suggests cardiac sympathetic activity. Except for LF/HF ratio, a definite decrease was observed in all the above considered measures of both time domain and frequency domain parameters in both the groups with T2DM, when compared to non-diabetics (Table 3 and figure 1,2,3,4). Studies using heart rate variability in a large population have demonstrated similar changes of reduced heart rate variability measures, as observed in this study. In the Framingham Study of 1919 individuals, HRV, as measured using SDNN, LF, HF, and LF/HF from 2-hour recordings, was also lower among diabetic subjects. [14]
- It was also observed while analyzing correlation between duration of diabetes and LF power of HRV test parameter, that there was non-significant correlation in less than 8year T2DM group while there was significant negative correlation in more than 8year group (Table4 and 5). This trend shows the late involvement of cardiac sympathetic fibres.
- LF/HF ratio parameter of HRV test depicts sympathovagal tone, so it is pertinent to note that there was increase in value of LF/HF ratio in T2DM patients of less than 8years group, as compared to non diabetic as well as T2DM of more than 8years (figure 5 and 6). This implies a greater decrease in HF power (depicting parasympathetic activity) than LF power (depicting sympathetic activity) in less than 8years of T2DM, showing early damage of parasympathetic fibres. This is also seen in this study [15], [16]. There was significant change in LF/HF ratio in both the said groups when compared to non-diabetics. Also, there was significant change in mean values of LF/HF ratio between T2DM patients of less than 8years and T2DM patients of more than 8years.
As already stated by American Heart Association, heart diseases and stroke are the No. 1 causes of death and disability among people with Type 2 diabetes. About 65 percent of people with diabetes die from heart disease and stroke according to National Diabetic Education Program. Moreover asymptomatic autonomic neuropathy is more common in Type 2 diabetes mellitus which is the main reason behind occurrence of painless myocardial infarction and sudden cardiac death in T2DM patients [17],[18]. The observations made above are suggestive of the fact that measurement of HRV may be used for early identification and prevention of cardiac complications in patients with DM. HRV test as an indicator of CAN is easy to perform and less time consuming. There is wide scope of involving HRV test as a routine screening for CAN. Regular HRV testing provides early detection and thus facilitates timely diagnostic and therapeutic interventions.
CONCLUSION:
Cardiac autonomic neuropathy is the major chronic complication of type 2 diabetes mellitus and one of the leading causes of cardiac diseases in diabetics. It is concealed for a long time before it reaches the stage of clinical diagnosis. Moreover, chronic hyperglycemia leads to sustained degeneration of autonomic nerves. This study has shown that Heart Rate Variability is a simple yet sensitive method of assessing cardiac autonomic neuropathy at an early stage in diabetics. This provides an opportunity so that proper lifestyle interventions and glycemic control can be undertaken to improve the condition of patient.
ACKNOWLEDGEMENT:
|
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
|
References:
[1]Fauci, A S, Braunwald E, Kasper D L, Hauser S L, Longo Dan L, Jameson J Larry (2008). Harrison's Principle of Internal Medicine 17 Edition.
[2] International Diabetes Federation. IDF diabetes atlas. 6th Brussels: International Diabetes Federation, 2013.
[3] Fauci, A S, Braunwald E, Kasper D L, Hauser S L, Longo Dan L, Jameson J Larry (2008). Harrison's Principle of Internal Medicine 17 Edition
[4] A. I. Vinik, R. E. Maser, B. D. Mitchell, and R. Freeman, “Diabetic autonomic neuropathy‚“ Diabetes Care, vol. 26, no. 5, pp. 1553–1579, 2003.
[5] R. E. Maser and M. J. Lenhard,‚“Cardiovascular autonomic neuropathy due to diabetes mellitus: clinical manifestations, consequences, and treatment,”Journal of Clinical Endocrinology and Metabolism, vol. 90, no. 10, pp. 5896–5903, 2005
[6] R. E. Maser, B. D. Mitchell, A. I. Vinik, and R. Freeman, “The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes a meta-analysis,” Diabetes Care, vol. 26, no. 6, pp. 1895–1901, 2003
[7] S. Tesfaye, A. J. M. Boulton, P. J. Dyck et al., “Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments,” Diabetes Care, vol. 33, no. 12, pp. 2285–2293, 2010.
[8]. American Diabetes Association, Diabetes Care 2015 Jan; 38 (Supplement 1); S8-S16
[9]. Kahn CR. Banting Lecture. Insulin action, diabetogenes, and the cause of type II diabetes. Diabetes 1994. Aug?43(8):10661084.
[10]. Robertson RP. Antagonist: diabetes and insulin resistance-philosophy, science, and the multiplier hypothesis. J Lab Clin Med 1995. May?125(5):560564, discussion 565.
[11] Task Force of the European Society of Cardiology and North American Society of Pacing and Electrophysiology: Heart rate variability: standards of measurements, physiological interpretation and clinical use. Circulation 1996; 93:1043–1065,
[12]. Acharya R, Hegde BM, Bhat PS, Rao A and Niranjan UC; Heart rate variability: a review. Med BiolEngComput.2006 Dec;44(12):1031-51. Epub 2006 Nov 17.
[1]]. Akselrod S, Gordon D, Ubel FA, Shannon DC, Barger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat to beat cardiovascular control. Science.1981;213:220-222
[14]. Singh JP, Larson MG, O'Donnell CJ, Wilson PF, Tsuji H, LloydJones
DM, et al. Association of hyperglycemia with reduced heart rate variability: The Framingham Heart Study. Am J Cardiol 2000?86:30912.
[15]. Kudat H, Akkaya V, Sozen AB, Salman S, Demirel S, Ozcan M, Atilgan D, Yilmaz MT, Guven O “Heart rate variability in diabetes patient” The Journal of International Medical Research 2006; 34: 291 – 296.
[16]. Spallone V, Bernardi L, Maiello MR, Cicconetti E, Ricordi L, Fratino P, Menzinger G Twenty-four-hour pattern of blood pressure and spectral analysis of heart rate variability in diabetic patients with various degrees of autonomic neuropathy. Comparison to standard cardiovascular tests; ClinSci(Lond). 1996; 91 Suppl: 1057.
[17] Neil HA, Thompson AV, John S, McCarthy ST, Mann JI. Diabetic autonomic neuropathy: the prevalence of impaired heart rate variability in a geographically defined population. Diabetic medicine : a journal of the British Diabetic Association. 1989;6(1):20-4.
[18]Murata K, Sumida Y, Murashima S, Matsumura K, Takeda H, Nakagawa T, et al. A novel method for the assessment of autonomic neuropathy in type 2 diabetic patients: a comparative evaluation of 123I-MIBG myocardial scintigraphy and power spectral analysis of heart rate variability. Diabetic medicine: a journal of the British Diabetic Association. 1996; 13(3):266-72. Epub1996/03/01.doi: 10.1002/(SICI)1096-9136(199603)13:3<266::AIDDIA72> 3.0.CO;2-4.









 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License