IJCRR - 10(4), February, 2018
Pages: 12-20
Date of Publication: 17-Feb-2018
Print Article
Download XML Download PDF
Analysis of Registered Cardiovascular Clinical Trials in India
Author: Ranjana Bharti, Narendra Singh
Category: Healthcare
Abstract:Background: Cardiovascular diseases (CVDs) are reported as the leading cause of death in the India. The landscape of CVDs clinical trials (CTs) are helpful in understanding the trends in progression of RandD and also offer insights of emergence of new technologies over a span of reporting years.
Method: Data was collected for CVDs CTs from database of clinical trial registry India (CTRI) by using keyword \"cardiovascular\" since 01/01/2008 to 31/08/2017. Data collected for each CTs includes health condition, clinical trial phase (phase I, II, III, IV), study design (randomized/non randomized), type of trials (interventional/observational), study type (drug/ayurveda/medical device/yoga/behavioral) institution type (public/private/partnership), sponsors and monetary support (domestic/international and government/industry/ academia/hospitals), publications (published/not published) and postgraduate thesis (yes/no) and year of registration. Result were analysed in terms of percentage of CTs i.e. n=239 using above mentioned indicators.
Results: The study found239 CTs of which majority of them were belong to Phase III (34%) and interventional (69%). Considering the study design, mostly CTs were randomized, parallel group, placebo controlled (25.9%) and conducted in hospitals (29%). For focused health condition, 17.5% associated with diabetes and 15.4% with coronary artery disease. From 2008, 15.8% postgraduate thesis trials had been registered with CTRI but non publication of CTs are highly prevalent. Numerous registered CTs was unclear phase (36%). With regard to type of study, CTs deals with drugs (81%), medical device (19.3%), Ayurveda, yoga, naturopathy and laughing therapy.
Conclusion: The CVDs CTs are low in India and poor access to treatment and out of pocket expenditure are key issues of CVDs management and there is need for effective and inexpensive care with indigenous radical change.
Keywords: Clinical trials, Cardiovascular, Clinical trial registry, India
DOI: 10.7324/IJCRR.2018.1043
Full Text:
Introduction
Cardiovascular diseases (CVDs) are major cause of the highest mortality and morbidity globally. According to WHO report, CVDs alone account for 26 percent of all deaths in 2014. Chronic respiratory diseases (CRDs), cancer and diabetes accounted for 13, 7 and 2 percent respectively [1].The potentially productive years of life lost (PPYLL) due to CVDs in the age group of 35-64 yrs. was 9.2 million in 2000 and is expected to increase 17.9 million in 2030 [2].Despite the high burden of CVDs, the trends in research and development (RandD) have not been analyzed systematically. The study deals with the analysis of evidence based practice (EBP) in the form of clinical trials (CTs) which provides significant information for understanding the progression of RandD and offer insights of emergence of new technologies in the field of CVDs. CTs possess huge potential for benefiting patients, improving therapeutic treatments and ensuring advancement in medical technology[3]. India is counted in a preferred destination for clinical trials due to its preponderance of high diseases and its genetically diverse population. A study by Mondal et al (2015) has analyzed that the low cost of clinical labors, availability of expert researchers, large market opportunities and presence of numerous clinical research organizations (CROs), make India an attractive destination for conducting CTs[5]. The paper also investigate the active player involved, focused health condition and nature of RandD on CVDs in India. The “Declaration of Helsinki” in World Medical Association (WMA) 2013, states that “every research study involving human subjects must be registered in a publicly accessible database before recruitment of the first subject” [4]. Drug controller general India (DCGI) made it necessary to register all CTs in clinical trial registry India (CTRI) before enrollment of participants, whether they belong to any category i.e. drugs, vaccine, therapy, Ayurveda, preventive measures, yoga based, naturopathy, behavioral, nutraceuticals, medical device, surgical methods, physiotherapy, observational as well as trials being conducted in the purview of the department of AYUSH. CTs registration will certify responsibility, transparency and availability of trials [3]. As per June 2017, CTRI has registered 8950 trials, out of which 3318 are prospective and 5604 are retrospective registrations [4].
Method and Materials
Data sources and extraction: The study is based on secondary data source. Data was collected for CVDs CTs from database clinical trial registry India (CTRI) maintained by Indian council of medical research (ICMR) by using keyword “cardiovascular” during the period of01/01/2008 to 31/08/2017.Data collected for each CTs includes health condition, CTs phase (phase I, II, III, IV), study design (randomized/non randomized), type of trials (interventional/observational), study type (drug/ayurveda/medical device/yoga/behavioral), intervention, institution type (public/private/partnership), sponsors and monetary support (domestic/international and government/industry/ academia/hospitals), publications (published/not published) and postgraduate thesis (yes/no) and year of registration. Descriptive analyses was used in the study and result were analysed in terms of percentage of total CTsusing above mentioned indicators.
Results
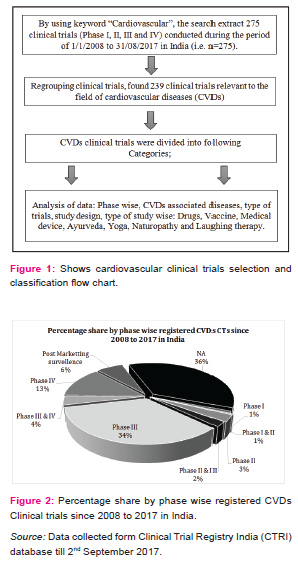
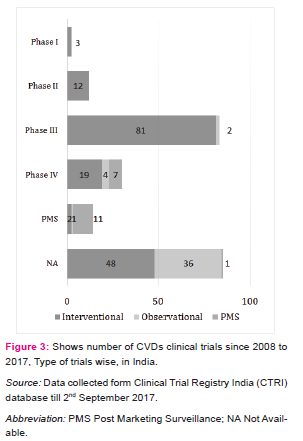
Percentage share by phase wise registered CVDs CTs
By using keyword “cardiovascular”, the search extract 275 CTs registered during the period of 01/01/2008 to 31/08/2017 in which 239 CTs have been found relevant to the field of CVDs. Figure 2 shows the percentage share by phase wise registered CVDs CTs in India. The phase I is dedicated to test new drugs or new forms of treatment in a small group of healthy volunteers to test the safe dose for the drug and its pharmacokinetics study, specifically in the country where the drugs or new chemical entity (NCE) was originally developed and then outsources to other countries [5][6], but very less number of CTs are going in this phase and almost similar results were seems in phase II CTs, also called as exploratory trial. This exploratory phase is carried out to test the efficacy of drug for particular disease and to explore and collect more information regarding side effects and doses with maximum efficacy. As shown in figure 2, the phase III is attracted maximum CTs industry (34%). This phase is also called confirmatory trials and conducted to confirm and obtain sufficient data regarding efficacy and therapeutic dose of the drug on larger group (approximately 1000 - 3000) against standard treatment. Drug development technology is not involved in this phase and as the above mentioned figure show in India, larger number of CTs are going on in this phase and very few industry undertake phase I. The phase IV trials are known as post marketing surveillance (PMS). This phase is conducted upon wide numbers of people after the drug is made available to doctors. The practitioners start prescribing drugs against specific clinical condition and observe the long term benefits and risk of the medicine and also gather information about its safety and side effects [6] [7].
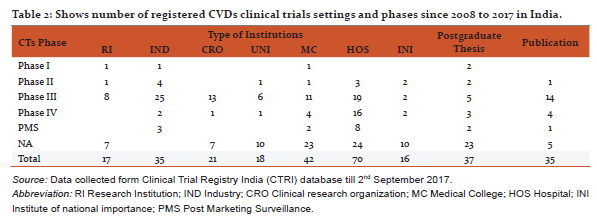
Characteristics of registered CVDs CTs
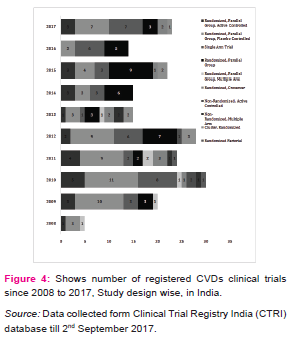
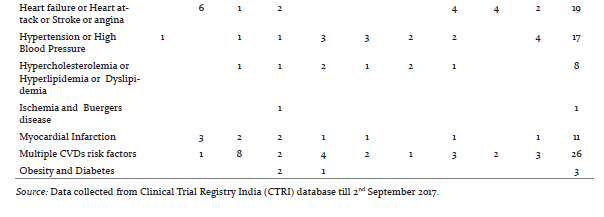
CVDs are considered as life style diseases or high burden diseases of India and shares some common risk factors including use of tobacco and excessive alcohol consumption, stress, physical inactivity, unhealthy diet, working nature of the people and other environmental factors [8], it leads to high blood pressure, obesity, raising blood sugar level, increased blood lipid content etc. Thus, CVDs is associated with many diseases (Table 1) e.g. diabetes, hypertension, obesity, COPD etc. The maximum CTs are being conducted in the condition of CVDs with diabetes (17.5%) and coronary artery disease (15.4%). Table 2 shows registered CVDs CTs settings and phases, it represents phase of CTs, type of institutions, number of publications and postgraduate thesis. To explore the results, Hospitals conducted large number of CTs (29%), Medical colleges (17.5%), Industry (14.6 %), CROs (8.7%) and then university (7.5%) and research institutions (7.1%) respectively. Organizations are taking advantage of large patients pool, highly skilled medical researches, low cost of drug development and timely completion of CTs in India, and CROs are also expanding their programs in India due to these attractive benefits [5].The total number of 239 CTs has been found to be concluded and out of which only few have been reported to be published (35/239, 14.6%).After completion of any CT, documentation in the form of publication is very essential, as it may use for further research and evidence based practices. Many CTs were being conducted for postgraduate thesis(38/239, 15.8%) but it seems that, most of the CTs are also not published and this highlight need of increasing awareness regarding documentation of CTs among CVDs researchers and academician. Other than this, numerous CTs was unclear phase (87/239, 36%) in registered CVDs CTs. Considering the type of trial, most of the CTs were interventional study (165/239, 69%) while only 43/239 (17.9%) were observational study (figure 3). Similarly, in study design, maximum number of registered CVDs CTs were randomized, parallel group, placebo controlled (62/239, 25.9%), single arm trial (39/239, 16.3%) and randomized, parallel group (38/239, 15.8%) respectively. CTs are need of the hour and randomized controlled trials (RCTs) are the important standard for establishing the advantage of a new treatment over an existing standard treatment (placebo) [9].
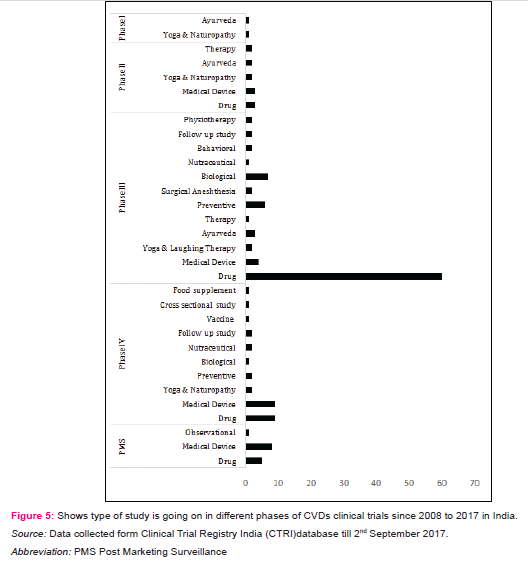
Percentage share by type of study in registered CVDs CTs
The effective management of non-communicable diseases (NCDs) or CVDs, demands periodic checkups,long term monitoring and life time treatments with drugs. The study found four major categories on which CVDs CTs going on in India from 01/01/2008 to 31/08/2017; Drugs and Vaccines, Medical devices, Ayurveda, Naturopathy, Yoga and laughing therapy (figure 5).
Drugs and Vaccines
Drugs are act as backbone in CVDs treatment. The study found74 CTs are on drugs, in which maximum number of CTs are going in phase III (81%) by Industry, hospitals, clinical research organizations and medical colleges (Table 3). Many CTs were belong to study of aliskiren (monotherapy or combination; dosage variation)and Serelaxin for myocardial infarction. alirocumab for hypercholesterolemia; aspirin and rivaroxaban for venous thromboembolism; aleglitazar, rivaroxaban, darapladib, lixisenatide and atorvastatin for acute coronary syndrome; azilsartan medoxomil for hypertension; canagliflozin on renal and CVDs in participants with diabetic nephropathy etc. CVDs research with concomitant diseases i.e. diabetes mellitus, obesity, congestive heart failure are very essential for effectively manage the disease. One of the institute is also studying the role of influenza vaccine in reducing heart attack, stroke, hospitalization and death in patients of heart failure.
Medical device
The medical devices plays very essential part in CVDs treatment. Phase II, III and IV includes 19.3% CTs are going on medical devices to treat aortic valve stenosis and coronary artery disease. The CTs deals with evaluation of sirolimus eluting bioresorbable vascular scaffold system (BVS), daffodil pericardial bioprosthesis, BioMime™ - sirolimus eluting stents, NUVANT MCT system external loop record and hydra trans aortic valve etc. Analysis on blood clot related complications between current generation valve vs previous generation valve are also carrying out.
Ayurveda
Ayurveda is one of the alternative and significant system of medicine requiring generation of high quality evidence for rational practice [11]. The analyses found very few CTs evaluating ayurveda for CVDs e.g. efficacy of hridayarnava rasa on hyperlipidaemia, effects of barley flour consumption and effect of Black seeds (nigella sativa) on diabetes and heart related illness etc.Another CTs is going on healthy aged people by giving an ayurvedic herbal extracts drug to see its effects on enhancing and maintaining healthy state.
Yoga, Naturopathy and laughing therapy
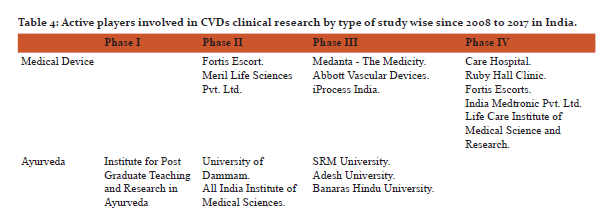
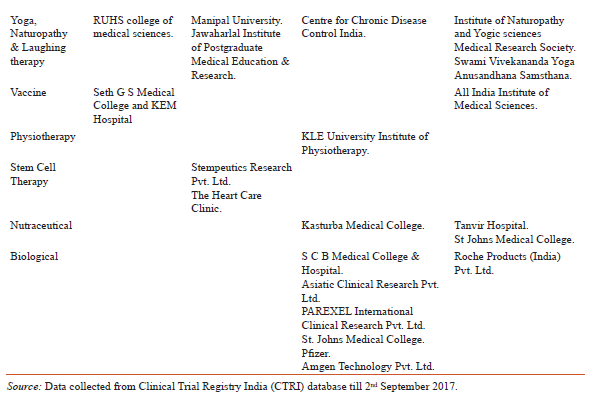
In addition to the incorporation of treatment by drug, vaccine, medical device and Ayurveda, yoga is also an important member to prevent and treat lifestyle diseases or CVDs. Yoga has been described as action leading to the union of the body and the mind which improves physical, mental and spiritual well-being [10].The study found 12 CTs in different phases and all were linked with either laughter therapy or naturopathy. The findings deal with cardiac rehabilitation programs, effect of yoga on patients going in heart surgery and after heart attack, effect of yoga on lung, heart functions and stress in breast cancer patients undergoing radiotherapy and effect of naturopathy and yoga on inflammation and utilization of insulin in patients with high risk of CVDs and effect of functional foods, yoga and laughing therapy in hypertensive women.Table 4 is showing active players involved in the clinical research for medical devices, ayurveda, yoga, laughing therapy, naturopathy and vaccine. In addition physiotherapy, biologicals, nutraceuticals and stem cell therapy are also important areas in which cardiovascular CTs are going on and might be helpful in exploring the new ways to deal with CVDs in India.
Discussion
Registered CVDs CTs during the period from January 2008 to August 2017 were recognised and analysed. The study observed that very low number of CVDs CTs are going on in India. CVDs are associated with many diseases e.g. high blood pressure, obesityand also responsible for leading cause of diabetes related morbidity and mortality [12]. Yoga and laughing therapy are also considered as effective way to deal CVDs [13] but very less number of interventions were found in these categories. Therefore, in the view of above, it can be said that to treat and explore new methods to eradicate the disease and maintain healthy lifestyle, there is an urgent need of less expensive, innovative modes for reducing CVDs and its associated complications through clinical interventions and public health policies.
Conclusion
Despite the high burden NCDs, the study found less number of CTs (n=239) being conducted in the field of CVDs, although an increasing trend over a span of years. Indian active players are mainly focused on Phase III CTs that involve testing of established drugs and minimize participant’s risk. Large patient’s pool, highly skilled medical researchers, low cost of drug development and timely completion of CTs made India an attractive destination of CTs but there is also need to increase the phase I and phase II CTs and come up with the essential changes for effective CVDs management. Most of the CTs were interventional, randomized, parallel group, placebo controlled and were conducted in hospitals. Non-publication of CTs in CVDs research are highly prevalent and large number of CTs had an unclear phase. Furthermore, the type of study found many CTs on drugs to assess the safety and efficacy and also includes analysis by monotherapy or combination; dosage variation of drugs. Even though poor access to drugs, medical device and out of pocket expenditure are key issues of CVDs management and the demand for effective and inexpensive treatment for CVDs will exert major pressure on Indian healthcare system. Additionally, very less number of CTs are found in Ayurveda, yoga, naturopathy and laughing therapy. These categories are also considers as an important member to prevent and treat lifestyle diseases or CVDs. In this context, CVDs research should be concentrated on primary prevention, effective and inexpensive health care services, epidemiology and clinical research.
Acknowledgement
Author acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The author is also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed. The author is thankful to Central University of Gujarat (CUG), Gandhinagar for infrastructural support and University Grant Commission (UGC), Government of India for fellowship support. The author is also grateful to her research supervisor Dr. Parvathi. K. Iyer, Asst. Professor, CUG, Gandhinagar for constant guidance and encouragement.
Source of funding No funding source
Conflict of interest No conflict of interest.


References:
- Non communicable country profiles 2014. World Health Organization (WHO) ISBN 9789241507509.2014.
- Shah J. “Prevention is Better than Cure”. Konnect Health. [Cited 2017 Dec].
- Clinical trial registry India (CTRI). National Institute of medical statistics. Indian council of medical research (ICMR). [Cited 2017 Dec].
- CTRI Bulletin. Clinical trial registry India (CTRI). National Institute of medical statistics. Indian council of medical research (ICMR). 2017; 12.
- Mondal S, Abrol D. Clinical trial industry in India: A systematic Review. Working paper 179. Institute for studies in industrial development (ISID).
- Bajpai V. Rise of clinical trials industry in India: An analysis. Hindawi Publishing Corporation. ISRN Public health. 2013. Article ID 167059 (17).
- Saxena P, Saxena R. Clinical Trials: Changing Regulations in India. Indian J Community Med. 2014; 39(4): 197–202.
- Mohan S, Reddy S. Chronic diseases in India: Burden and Implications. Risk Dialogue Magazine. Swiss Re Centre for Global Dialogue. 2014 [Cited 2016 May].
- Kabisch M, Ruckes C, Grafe M S, Blettner M. Randomized controlled trials: part 17 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2011; 108(39): 663–668.
- Mehta JL, Mehta P, Pai BV. Yoga and Cardiovascular Disease. J Yoga and Physio. 2017; 3(1): 555604.
- Sridharan K, Sivaramakrishnan G. Clinical trials in Ayurveda: Analysis of clinical trial registry of India. Journal of Ayurveda and Integrative Medicine 2016;7:141-143.
- Leading causes of diabetes related morbidity and mortality. Circulation. American Heart Association, Inc. 1999.
- Neto M G, Rodrigues E S, Silva W M, Carvalho V O.Effects of Yoga in Patients with Chronic Heart Failure: A Meta-Analysis. Arq Bras Cardiol. 2014; 103(5):433-439.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License