IJCRR - 10(1), January, 2018
Pages: 22-26
Date of Publication: 10-Jan-2018
Print Article
Download XML Download PDF
Studies on In-Vitro Interaction of Artemether-Lumefantrine with Food Components
Author: Awofisayo Sunday O., Arhewoh Matthew I., Okhamafe Augustine O.
Category: Healthcare
Abstract:This work evaluates in vitro interaction of food components with artemether\?lumefantrine (AL) in tablet. Pelletized food components (i.e., starch, albumin, sunflower oil and carbonated drink) with AL tablet were observed using Fourier transform infrared (FTIR) spectroscopy while spectra obtained were compared using essential FTIR (eFTIR) software. The influence (i.e., instantaneous change in pH (IPH) and acid buffering capacity (ABC)) of food components on Fasted State Simulated Gastric Fluid (FaSSGF) and Fed State Simulated Intestinal Fluid (FeSSIF) were assessed alongside dissolution kinetics of actives in AL tablets. Artemether and lumefantrine produced identical spectra features with reference infra red (IR) spectra. No change in spectra features of AL on carbohydrate, vitamin A and oil treatment but noted with albumin and the carbonated drinks. IPH values for albumin revealed statistical higher value (p < 0.05) than other food components in FaSSGF. ABC for albumin treatment was statistically lower (p < 0.05) than for other food components in FaSSGF. Artemether overall release was significantly reduced by starch, lactose and carbonated drink (p < 0.05) while that of lumefantrine was significantly increased by sunflower oil (p < 0.05). There was interaction between AL and albumin, or carbonated drinks which can be of importance in drug treatment outcome.
Keywords: Artemether-lumefantrine; Food component; In vitro interaction; Dissolution kinetics; Instantaneous pH change, Acid buffering capacity
DOI: 10.7324/IJCRR.2018.1016
Full Text:
INTRODUCTION
The oral route is the most convenient for drug administration when treating uncomplicated Plasmodium falciparum malaria in the tropics. Consequently, a majority of World Health Organization (WHO) recommended drugs are presented as solid formulations for oral administration (Katzung, 2009). Artemether-lumefantrine (AL) is a co-formulated artemisinin combination therapy (ACT) available for paediatric and adult use and widely prescribed across sub-Saharan Africa (Katzung, 2009).
Factor that are critical to the kinetics of dissolution as presented by the Nernst Brunner and Levieh modifications of the Noyes-Whitney model include physiochemical properties of the dissolving compound (i.e., pKa, solubility, crystalline energy, specific surface area) and certain prevailing conditions of the gastrointestinal (GI) tract) (Kostewicz et al, 2002). Physiological parameters that play an important role include pH, surface tension and volume of the GI luminal fluid, solubilization and buffer capacity of substances present in the GI tract (Dressman et al, 2001). These parameters change following ingestion of food (Schmidt and Dalhoff, 2002). Amphiphilic bile components (i.e., bile salts and lecithin) may increase in concentration following a meal (Martinez and Amidon, 2002). Biliary secretion has been demonstrated to increase the in vivo dissolution rate for some poorly soluble compounds. In order to develop a predictive in vitro model to forecast the in vivo performance of drugs, the Fasted State Simulated Gastric Fluid (FaSSGF), Fed State Simulated Gastric Fluid (FeSSGF), Fasted State Simulated Intestinal Fluid (FeSSIF) and Fed State Simulated Intestinal Fluid (FeSSIF) were proposed and are now widely employed (Vertzoni et al, 2005). FeSSIF-V2 (version 2) was further advanced following its close compositional resemblance to intestinal fluid (Jantratid et al, 2008).
In the present study, the aim was to assess in vitro interaction of food components with AL tablet.
MATERIALS AND METHODS
Materials
AL tablet (Coartem®) was purchased in Uyo, Southern Nigeria. The food components were similarly purchased in Lagos, Nigeria. FaSSIF/FeSSIF/FaSSGF and FeSSIF-V2 powder were products of biorelevant.com, UK. Details of the drug and food components are presented in Table 1. Acetonitrile, tetrahydrofuran (THF) and potassium dihydrogen phosphate were of HPLC grade, products of Sigma Aldrich, Germany. All other reagents were of analytical grade.
Methods
Preparation of standard solution of internal standard, artemether and lumefantrine
Ten milligram of halofantrine was transferred into a 10 mL volumetric flask containing 6.0 mL of methanol to dissolve. The powder was dissolved and thereafter made up to mark to produce stock concentration of 1000 µg/mL. Reference standard powder of artemether 20 mg and lumefantrine 120 mg were weighed accurately into different flasks. Artemether and lumefantrine powder were dissolved to produce stock solutions of 2 mg/mL and 12 mg/mL, respectively.
Preparation of mixed standard solutions of AL and (IS)
Equal volumes (2 mL) of artemether and lumefantrine stock solutions were dispensed into 5 mL sample bottles to produce 1 and 6 mg/mL of the standards, respectively. Serial dilutions of the mixed standard and reference standard solutions were made to obtain graded concentrations of AL ranging from 0.01/10 to 2/10 mg/mL. Mixed standard solutions were prepared using acetonitrile and THF (50:50, %) as diluents. The resulting solutions were spiked with IS solution to give 5 mg/mL.
Preparation of hydrochloric acid/sodium chloride solution pH 1.6
A weight of 1.0 g of sodium chloride was dissolved in 0.450 L of distilled water. The pH was adjusted to 1.6 with HCl. Either HCl or NaOH were spiked as applicable to give the exact pH 1.6, thereafter made up to 0.5 L with distilled water.
Preparation of sodium hydroxide/glacial acetic acid/sodium chloride–Buffer solution pH 5
A weight of 2.020, 4.325, and 5.937 g of sodium hydroxide pellet, glacial acetic acid and sodium chloride, respectively, were dissolved in 0.450 L of distilled water. The pH was adjusted with either of 1N HCl or NaOH solution.
Preparation of FaSSGF, FeSSIF and FeSSIF–V2
A weight of 1.120 g of FaSSIF/FeSSIF powder was dissolved in 0.25 L of acid buffer (pH 1.6). This was stirred until the powder was completely dissolved. The solution was made to 0.5 L mark. A weight of 5.60 g of FaSSIF/FeSSIF/FaSSGF powder was dissolved in 0.25 L of buffer (pH 5.0). The solutions were made up to volume with the buffer and allowed to stand for 2 h before use. FeSSIF-V2 was prepared by dissolving 5.0 g of FeSSIF-V2 powder in 5.0 L of distilled water.
Spectroscopic determination
Infra red (IR) studies were performed using Fourier transform infra-red (FTIR) spectrophotometer, FTIR-84005 (Schimadzu, Japan). One milligram of AL powder and 200 mg of dried KBr powder were mixed and compressed into a tablet shaped disk and scanned at a speed of 2 mm/s over a wavenumber region from 4000 to 500 cm-1. Essential FTIR (eFTIR) software was employed for spectral analysis.
Dissolution kinetics of AL in the presence of FeSSIF-V2
A total weight of 2 g of each solid food components or 1.7 mL of sunflower oil or 35 cL of carbonated drinks was dispersed in 500 mL FeSSIF–V2 in a flask. One tablet of the AL product was subsequently placed in the dissolution apparatus; USP type 2.The dissolution profile was determined by sampling 5 mL of the medium at 0, 5, 15, 30, 45, 60 and 90 min. Samples were analyzed using HPLC system.
Statistical analysis
The data obtained were analyzed using Minitab for Windows statistical package (Minitab, Inc., USA). Post hoc comparison was performed with Hsu’s test.
RESULT
Albumin had a pH change of 1.51 units in FaSSGF (Table 2). Similarly, it increased the pH in FaSSGF from 1.62 to 3.13 units and revealed an increase of 0.17 units in FeSSIF. Albumin also demonstrated significantly lower (p < 0.05) ABC compared to the carbohydrates in FaSSGF (Table 2).The ABC of Cocacola® was significantly higher (p < 0.05) than Fanta®. Carbonated drinks treatment presented the highest ABC. The extent of artemether release in the presence of fructose was statistically higher than for starch (p<0.05). Albumin and fructose treatment had significantly higher total artemether release adjudging by the area under the curve (AUC) values (Table 4), (p < 0.05).
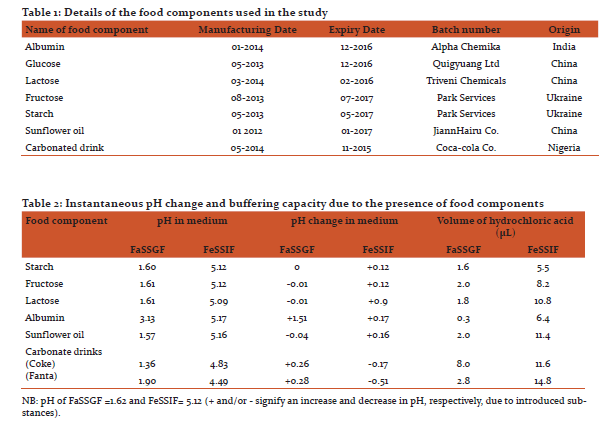
Dissolution efficiency (DE) values of 1.06 and 0.96, respectively for albumin and fructose treatments gave indication of satisfactory artemether release. Lumefantrine dissolution throughout the 90 min run time was less than 20% for the food components except sunflower oil with a value of 59.7% (Figures 1b and 2b).
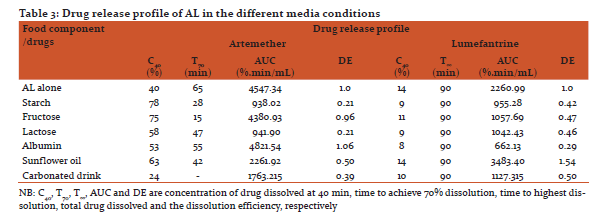
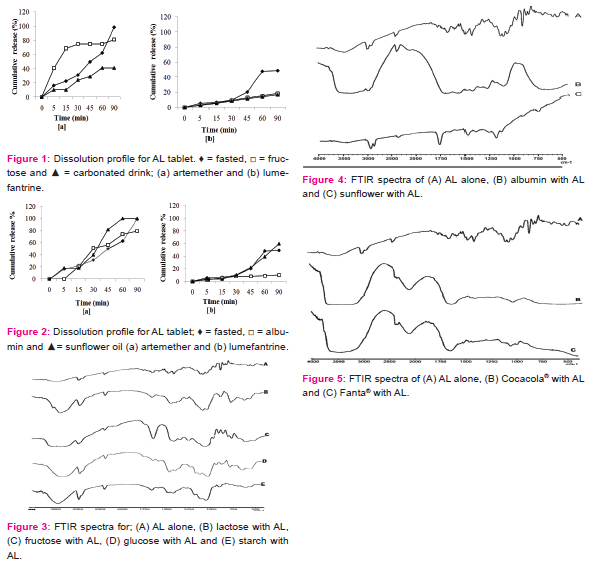
In AL spectra presented (Figure 3A), there were broad peaks for artemether aliphatic O-H stretching vibration and aliphatic C-H bending vibrations. Treatment with albumin (Figure 4B) and carbonated drinks (Figure 5B) showed deletion of the O-H bond vibration. Carbonated drinks further caused a deletion of the C-H, C-O and C-O-O bond vibrations. There were prominent C-H bending and O-H stretching vibrations for artemether in the AL spectra (Figure 4A) as well as the spectra for the food components treatments (Figure 3A-E and 4C), except for albumin (Figure 4B). The endoperoxide bond stretching was also deleted by carbonated drink treatment. The band due to C=O for carbonated drink treatment had wavenumber shifted downwards beyond 7 cm-1.
DISCUSSION
There are several reports on the impact of food and beverages on the luminal content physicochemical characteristics (Vertzoni et al, 2005; Kong and Singh, 2010). Foods with high protein content and high viscosity have been reported to cause higher gastric acid secretion (Marciani et al, 2001). This study assesses the instantaneous pH change and buffering capacity of food component and carbonated drinks in simulated gastric and intestinal fluids on the in vitro course of artemether and lumefantrine release from AL tablet.
The pH of the simulated media was altered instantaneously by food components to varying extent. Albumin showing a pH change of 1.51 units in FaSSGF is expected to present a significant change in the ratio of ionized to unionized drugs in solution. A measurable effect on the release profile of the components of AL tablets being studied is therefore expected. Carbohydrate and sunflower oil components did not reveal any significant change in pH of the two media studied.
Some studies have also reported that the pH of proximal jejunum increased to 6.5 after the administration of dried milk, vegetable oil and dextrose to healthy volunteers (Dressman et al, 1990). The buffering capacity of the different food components and drugs evaluates the influence of these agents on postprandial hydrochloric acid secretion in the stomach following the ingestion of drug doses concomitantly with food (Parapini et al, 2008). In this study, albumin increased the pH in FaSSGF sufficiently to affect the release profile of actives. Similarly, albumin had a pH increase of 0.17 units in FeSSIF. The effect of temporal changes in GI tract pH profile and the physicochemical basis of altered pH effect on drug absorption have been reported (Charman et al, 1997). The findings of this study are in agreement with those of Charman and co-workers with respect to the manifestations of the physicochemical properties of the studied drugs (i.e., food component in FeSSIF-V2) which may affect their bioavailability. Treatment with sunflower oil increased the in vitro artemether and lumefantrine release when compared with the other food components (Figures 1 and 2). This establishes the fact that lumefantrine possess some intrinsic aqueous solubility problems (Premji et al, 2008).
FTIR spectra of AL in this study (Figure 3) revealed frequencies that were consistent with the vibrational data reported by earlier studies of artemisinin and artesunate (Lawal et al, 2012). This study revealed that FTIR spectrum of AL conformed to the eFTIR analysis of the important bonds/functional group bands (Socrates, 1994; Sohrabi et al, 2005). The positions of these peaks in the food component treatment formed the basis for the assessment of possible food-drug interaction. AL treatment with albumin (Figure 4B), and carbonated drinks (Figure 5B) showed deletion of the O-H bond vibration. This is suggestive of molecular interaction in the albumin treatment. Deletion of certain characteristic bond vibrations of a molecule due to the presence of additives are marks of molecular interaction (Koutsoupakis et al, 2002). Spectra band due to C=O for carbonated drink treatment had wavenumber shifted downwards beyond 7 cm-1, which is strong indication of molecular interaction.
Spectra due to starch treatment were similar to those of other food components (except albumin) with no significant differences in the wavenumber when compared with the reference.
CONCLUSION
Carbonated drinks have significantly reduced the dissolution profile of artemether and lumefantrine from tablet. Starch resulted in good drug release for artemether but poor lumefantrine release profile.
AUTHOR’S STATEMENT
The authors have no conflict of interest to disclose.
ACKNOWLEDGMENT
The authors express their appreciation to F.I. Sanni for her assistance in procuring the biorelevant media powder used, and P.D. Ojobor for his technical assistance.
References:
- Katzung BG. Basic and Chemical Pharmacology 11th Ed McGraw – Hill; 2009.
- Premji ZA, Abdulla S, Ogutu B, Ndong A, Falade CO, Sagara I. 2008. The content of African diets is adequate to achieve optimal efficacy with fixed dose artemether – lumefantrine: a review of the evidence. Mal J, 7:244-9
- Kostewicz ES, Branus U, Becker R, Dressman JB. 2002. Forecasting the oral absorption behaviour of poorly soluble weak bases using solubility and dissolution studies in biorelevant media. Pharm Res, 29:345 – 50.
- Dressman J, Butler J, Hempenstall J, Peppas C.2001. The BCS: Where do we go from here.” Pharm Technol, July; 68-76.
- Schmidt LE, Dalhoff K. 2002. Food – drug interactions. Drugs, 62: 1481 – 502.
- Martinez MN, Amidon GL. 2002. A mechanistic approach to understanding the factors affecting drug absorption: A review of the fundamentals. J Clin Pharmacol, 42:620-43.
- Vertzoni M, Dressman JB, Butler J, Hempenstall J, Reppas C. 2005. Simulating of Fasting gastric conditions and its importance for the in vivo dissolution of hydrophilic compounds. Eur J Pharm Biopharm, 60: 413 – 7.
- Jantratid E, Janssen N, Reppas C, Dressman JB. 2008. Dissolution media simulating conditions in the proximal human gastrointestinal tract: an update. Pharm Res, 25: 1163 –76.
- Kong F, Singh RP.2008. Disintegration of solid food I human stomach. J Food Sci 73: R67-80.
- Marciani L, Gowland PA, Spiller PC, Manoy P, Moore RJ, Young P, Fillery-Travis AJ. 2001. Effect of meal viscosity and nutrients on satiety, intra-gastric dilution and emptying assessed MRI. Am J Physiol Gastroint Liver Physiol, 280:G1227-33.
- Dressman JB, Berardi RR, Dermentzoglou LC, Ressel TL, Schmaltz SP, Barnett JL, Jarvenpaa KM. 1990. Upper gastrointestinal (GI) pH in young, healthy men and women. Pharm Res, 7: 751-56.
- Charman WN, Porter CJH, Mithani S, Dressman JB. 1997. Physicochemical and physiological mechanisms for the effects of food on drug absorption: The role of lipids and pH. J Pharm Sci, 86:269-80.
- Parapini S, Olliari P, Navaratnam V, Taramelli D, Basilico N. 2008. Stability of the antimalarial drug dihydroartemisinin under physiologically relevant conditions: implications for clinical treatment and pharmacokinetic and in vitro assays. Antimicrob Agents Chemother, 59: 4046-52.
- Premji ZA, Abdulla S, Ogutu B, Ndong A, Falade CO, Sagara I. 2008. The content of African diets is adequate to achieve optimal efficacy with fixed dose artemether – lumefantrine: a review of the evidence. Mal J, 7:244-9.
- Lawal A, Umar RA, Abubakar MA, Faruk UZ, Wali U. 2012. FTIR and UV-visible spectrophotometric analysis of artemisinin and its derivatives. J Pharm Biomed Sci, 24: 6-14.
- Socrates G. 1994. Infra-red Characteristic Group Frequencies: Tables and Charts: John Wiley and Sons, New York, ISBN: 9780471942306, Pp: 150-290.
- Sohrabi MR, Davallo M, Tadayyon F, Nabipoor F, Khamneifar A. 2005. Simultaneous determination of acetylsalicylic acid and acetaminophen in ASA tablets by FTIR/ATR spectrometry with multivariate calibration data treatment. Asian J Chem, 17: 541-7.
- Koutsoupakis C, Giaolou I, Paulidou E, Kapelanaki C, Varotsis C. 2002. Antimalarial endoperoxides: synthesis and implications of the mode of action. ARKIVOC, 13: 62-9.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License