IJCRR - 9(24), December, 2017
Pages: 48-54
Date of Publication: 26-Dec-2017
Print Article
Download XML Download PDF
Study of Breast Lesions with Special Reference to Rare Malignant Epithelial Tumors in a Tertiary Care Hospital with Brief Review of Litreature
Author: Sheema Sheikh, Rohi Wani, Isma Niyaz, Farzana Manzoor, Lateef Ahmed Wani, Arshi Beg
Category: Healthcare
Abstract:Background and Aim: Lesions of the breast vary from completely benign Fibroadenomas to Invasive Carcinomas No Special Type (NST) and rare malignant lesions. Breast cancer is ranked as number one cancer among females in India. A study was carried out to know about histopathological spectrum of breast lesions with particular emphasis on rare malignant breast tumors.
Methods: A total of 568 surgical specimens of breast tissue, subjected to histopathological examinations in our tertiary care hospital over a period of 2½ years were taken up for study and their clinical and microscopic details were noted. All the cases were tabulated as benign and malignant categories. Cases were stratified according to latest, 2012 WHO classification of tumors of breast. Among the tumors, 357(75.31%) were benign and 117(24.68%) were malignant. Out of the 357 benign tumors, 289 were fibroadenomas and 11 were benign phyllodes tumor. Among the various malignant tumors, Invasive carcinoma of no special
type (NST) was the commonest malignancy (79.48%). Almost all rare types of malignancies were encountered like, Carcinoma with neuroendocrine differentiation, Medullary carcinoma, Invasive lobular Carcinoma (ILC), Tubular carcinoma and Metaplastic Carcinoma with no special type. The incidence of these rare breast cancers in our study is almost same as many other studies. Future extensive studies and for longer time period are needed to confirm the exact incidence of these tumors since these tumors have different prognostic and therapeutic considerations when compared with the common breast epithelial malignancies.
Keywords: Breast cancers, Invasive carcinomas no special type
DOI: 10.7324/IJCRR.2017.92410
Full Text:
Introduction
Breast cancer is the most common female cancer in the world with an estimated 1.67 million cancer cases diagnosed in 2010¹. In India, also Breast cancer is ranked number one cancer among females² with age adjusted rate as high as 25.8 per 100,000 women with the mortality of 12.7 per 100,000 women³,4. The histopathological type is mainly Invasive carcinoma of no special type (NST) which is 70-80%, followed by Invasive lobular carcinoma (5-15%) 5. However, there are rare breast tumors (<2% of all breast cancers) like Mucinous carcinomas, Malignant phyllodes, Neuroendocrine tumors which have particular prognostic or clinical characteristics 6. In our study of 2½ years, we have studied the histopathological and clinical spectrum of breast lesions in our department with special emphasis on rare malignant tumors. A brief review of literature is also done.
Material and Methods
All the breast specimens submitted for histopathological examination over a period of 2½ years (Jan 2015-June 2017) were taken up for this study. It is a Descriptive type of study. All the breast cases which included Lumpectomy, Excision biopsies, Tru cut biopsies, Mastectomy specimens and Blocks for Review were included in the study irrespective of age and sex. There were no exclusion criteria. Specimens were received in 10% formalin and were subjected to routine Hematoxylin and Eosin stains. All the sections were studied in detail, clinical history was noted down and morphological diagnosis was made.
Results
A total of 30,926 specimens were received in the surgical pathology section of the department of pathology in our Tertiary Care Hospital from Jan 2015–June 2017. Out of these 30,926 specimens, 568 were breast tissue specimens. All these surgical specimens constituted Lumpectomy specimens, mastectomy specimens, mastectomy specimens with axillary lymph node dissection and Tru cut biopsies. In some cases we received wax blocks for review. The various lesions encountered have been depicted in the tables.
Maximum cases, 542 were from females and 26 cases were from the male breast making the Male:Female ratio of 1:20.84. Most of the specimens from males were that of Gynaecomastia however there were 3 cases of carcinomas from male category.
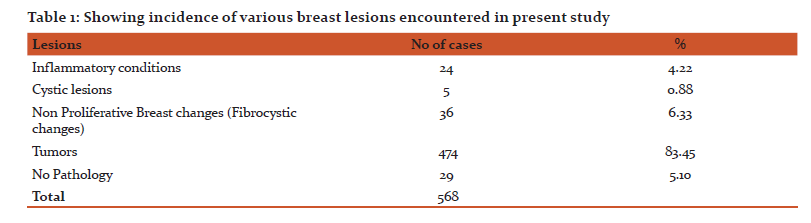
As shown in the Table 1, 24 (4.22%) inflammatory lesions, 36 (6.33%) fibrocystic changes, 5(0.88%) cystic lesions and 474(83.45%) tumors were encountered. No pathology was found in 29 cases. Among the inflammatory lesions, most of these were of abcesses, chronic inflammations and chronic granulomatous mastitis. There was a single case of Plasma cell mastitis also.
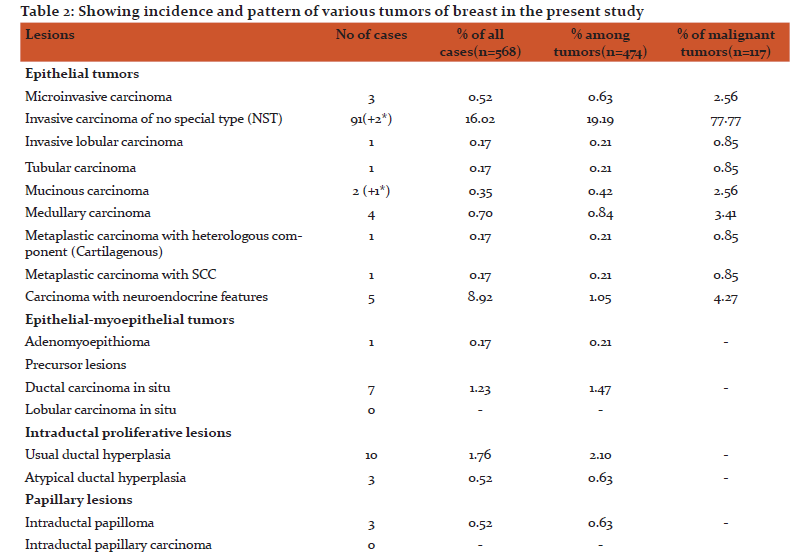
Among the tumors, 357(75.31%) were benign and 117(24.68%) were malignant. Out of the 357 benign tumors, 289 were fibroadenomas and 11 were benign phyllodes tumor. Among the various malignant tumors (Table 2), invasive carcinoma of no special type (NST) was the commonest malignancy (79.48%). Five cases of Carcinoma with neuroendocrine differentiation and four cases of Medullary carcinoma were seen. Only one case of each, Invasive lobular Carcinoma (ILC), Tubular carcinoma and Metaplastic Carcinoma with no special type were seen.
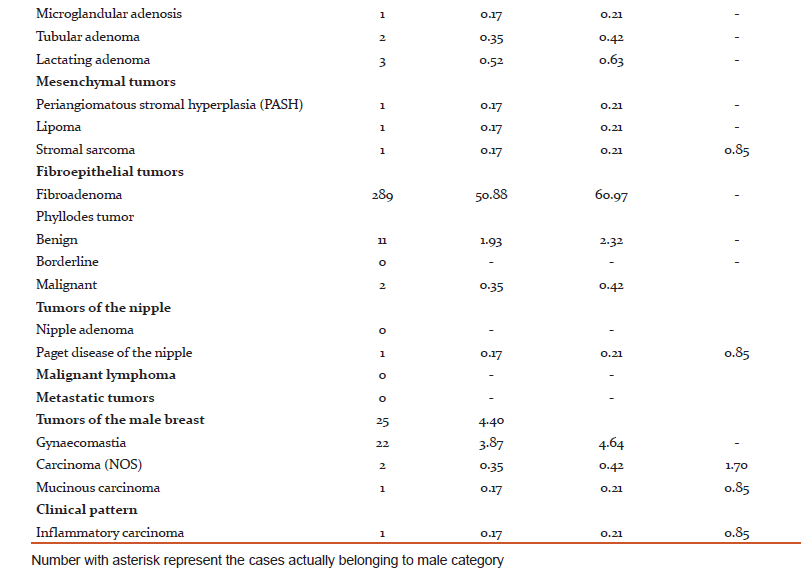
Of the various stromal tumors encountered, 11cases of benign phyllodes and 2 cases of Malignant Phyllodes were seen. There was a single case of Fibrosarcoma also. Among the rare benign lesions, one Periangiomatous Stromal Hyperplasia and one Adenomyoepithelioma were found.
Lesions in male breast were mostly Gynaecomastias (88%), however there were 2 cases of Invasive carcinoma NST and 1 Mucinous carcinoma.
There were 29 cases which were reported as unremarkable breast tissue or no pathology found. These cases were actually cases of axillary breast tissue, reduction mammoplasty in a male, or cases with no residual tumor or no recurrence.
Discussion
Breast cancer is the second most common cancer in the world, the first being the lung cancer. Incidence rates vary nearly fourfold across the world regions, with rates ranging from 27 per 100,000 in Middle Africa and Eastern Asia to 96 per 100,000 in Western Europe1. We studied a total of 568 breast lesions and found some interesting and rare malignant epithelial tumors. We have focussed on clinical presentation, detailed gross examination and histopathological analysis, although Immunohistochemistry (IHC) for Estrogen Receptor(ER), Progesterone Receptor(PR), Her-2 was done routinely, some special IHC markers like Non Specific Enolase(NSE), Chromogranin etc were also done wherever needed. In this study our main focus was simple macroscopic and microscopic features of rare epithelial breast malignancies.
In our study we came across almost all kinds of rare epithelial breast malignancies however we had only one case of Invasive lobular breast carcinoma(ILC) constituting 0.85% of malignant tumors which is quite low. Another interesting case of Mucinous carcinoma in male breast was also encountered. We will discuss the rare malignancy cases one by one in the following paragraphs.
Medullary carcinoma cases. Four cases of medullary carcinoma were seen in our study. The first was a 60 years old female who had presented with 2x2 cms freely mobile lump in upper quadrant of left breast for a month for which lumpectomy was done. Grossly, the mass measured 2.3x2x0.5 cms with grey cut section. No lymphnodes were resected. The second case was a 40 years old female presenting with lump right breast in the upper outer quadrant measuring 3x2 cms for 3 months and enlarged axillary lymph nodes for which mastectomy with axillary lymph node dissection was done. Grossly, breast measuring 18x11x5 cm with axillary tail measuring 8x5x2.5 cms was received. Cut section showed 2.5x2x3 cm, grey white growth. Twenty lymph nodes were dissected out, out of which one lymph node showed the metastasis of tumor present. The third case was that of a 38 years old female presenting with hard breast lump in the outer inferior quadrant of left breast for three months, was diagnosed as Medullary carcinoma on FNAC preoperatively. So a breast conservation surgery with axillary lymph node dissection was done. Grossly skin covered 8x4.5x2.3 cm breast tissue was received, cut section showed1.8x1x1 cm grey white growth. All the 14 Lymph nodes dissected out were free from tumor.
Medullary carcinoma constitutes less than 5% of tumors in most series of breast cancer.7,8 The mean age of patients with medullary carcinoma ranges from 42-52 years9,10 . Medullary carcinomas are diagnosed classically when there is a syncitial growth pattern of tumor cells constituting >75% of tumor, absence of glandular structures, diffuse moderate to marked lymphoplasmacytic infiltrate, moderate to marked pleomorphism and complete histological circumscription. A tumor may be classified as atypical when upto 25% of it is composed of Invasive carcinoma and the rest is classical Medullary carcinoma. The 4th edition of WHO classification of Tumors of the breast recommend that Classic (Medullary carcinoma), Atypical (Medullary carcinoma) and Invasive carcinoma NST with Medullary features be grouped with the category of Carcinoma with Medullary features11. Medullary carcinomas occur at high frequency in patients with BRCA-1 gene mutation. These typically lack ER and PR expression as well as HER-2 amplification (triple negative) and are usually highly proliferative and apoptotic tumors12,13.
The prognosis of patients with small node-negative medullary carcinoma is particularily favourable with a disease free survival of 90% or better10,14. Several studies have confirmed the favourable prognosis of Medullary carcinoma7,10,15. Medullary carcinoma has been reported to have better prognosis than Invasive carcinoma NST9,10, but this has been questioned by some studies16,17. Differences in diagnostic criteria may account for disparity10,15,16. Strictly defined diagnostic criteria may account for disparity10,15,16. Strictly defined morphological criteria are necessary to preserve the entity of Medullary carcinoma characterised by relatively favourable prognosis which is not shared by Atypical Medullary carcinoma18,19.
Carcinoma with Neuroendocrine features: Five cases of Carcinoma with Neuroendocrine features were encountered in our study. The first case was a 55 years old female with lump in right breast for which mastectomy with axillary lymph node dissection was done. Grossly we received 19x17x5 cm specimen of right breast which on cut section showed a friable growth measuring 5.5x5 cm. All the eleven lymph nodes were free from tumor. The second case was a 35 year old female with lump on left breast reported as Atypical fibroadenoma on FNAC for which excision (lumpectomy) was done. Grossly it was a globular fibrofatty tissue measuring 4.5x3x2.5 cm which on cut section showed a necrotic 3.5x3 cm grey white growth. The third case was a review case submitted as wax blocks for review in our department, she was a 40 year old female with lump in right breast. The 4th case was a 40 years old female with lump in right upper quadrant for which wide local excision and axillary lymphnode dissection was done. Grossly we received a skin lined soft tissue with cut section showing 2.5x2.2x2 cm growth with haemorrhage. All the 37 lymhnodes dissected out were free from tumor. The fifth case was 32 years old female with left breast lump for which lumpectomy was done. Cut section showed a friable growth.
Breast carcinoma with neuroendocrine differentiation (NE-BC) are relatively uncommon representing 1-2% of all breast cancers. The diagnosis requires at least one Neuro-endocrine marker (Chromogranin A or Synaptophysin) positivity in at least 50% of tumor cells. Those tumors with positivity of <50% of cells are reported to as breast carcinoma with NE morphology. NE-BC are more commonly seen in postmenopausal women older than 60 years20,21. Younger age group being reported from Asian countries22,23.Grossly NE-BCs are circumscribed and sometimes hemorrhagic with gray to tan cut surface. Microscopically the tumor has a nested, solid/papillary growth pattern with cells having relatively abundant cytoplasm often eosinophillic and granular. NE-BCs are usually strongly positive for ER and PR21. Data on clinical follow up of patients with NE-BC are linked with some studies showing low nuclear grade tumors having a very good prognosis24 whereas other studies25 show that they are relatively aggressive tumors.
Mucinous Carcinoma: Three cases of Mucinous carcinomas were encountered in our study. The first case was a 70 years old female presenting with lump right breast. We received lumpectomy specimen measuring 3x1.5x1 cm with cut section of greyish white to yellow. The second case was a 62 years old female with right breast lump measuring 2x2 cm and we received wax blocks for review in this case. The third case was a 45 year old male patient who presented with swelling left breast measuring 6x7 cm beneath nipple and areola for past 6 months. FNAC was done and it was reported as Breast Carcinoma NST. Tru cut biopsy was done and we received 4 cores of biopsy tissue ranging in size from 1-2 cm and it was reported as Mucinous Carcinoma.
Mucinous carcinoma also called Colloid, Mucoid or gelatinous carcinomas are tumors in which large amounts of extracellular mucin is often visible to naked eye. The mean age of patients with Mucinous carcinoma is reported to be older than with non-mucinous tumors26,27,28,29, being often over 60 years27,28. The incidence of pure mucinous carcinoma is 1-2% of all breast cancers27,30. These tumors are more frequent among Caucasian women31. Grossly the tumors are glistening, gelatinous with bosselated, pushing margins and soft consistency with a size range from 1-20 cm28,30,32. Microscopically they are characterised by proliferation of clusters of generally uniform, round cells with minimal amount of eosinophillic cytoplasm, floating in lakes of mucin. The diagnosis of pure mucinous carcinoma is usually made when more than 90% of the invasive component is admixed with stromal mucin. If stromal mucin is between 50-90%, the lesion is classified as mixed mucinous carcinoma. Most of the mucinous carcinomas are ER positive while <70% are PR positive.
Pure Mucinous carcinomas have relatively favourable prognosis26,27,30,31 being far better than mixed variety30,32,33 with at least 18% difference in survival rates in several studies.
Metaplastic Carcinomas: Two cases of Metaplastic carcinomas were seen in our study. The first case was an 80 years old female who presented with lump in right breast with enlarged axillary lymph nodes. Mastectomy with axillary lymph node dissection was done. On cut section whitish growth measuring 7x5x3 cm was seen. Nineteen lymph nodes were dissected out. Microscopy showed Infiltrating carcinoma NST with more than 50% of tumor showing morphology of moderately differentiated Squamous cell carcinoma component (Fig 1). The second case was a 36 years old female with mass right breast for which wide local excision was done. We received partially skin covered breast tissue with cut section showing a greyish white growth measuring 4x2.1 cm. Microscopically, Metaplastic carcinoma with heterologous cartilaginous element was seen (Fig 2). Metaplastic carcinomas are rare tumors and represent 0.3% of all invasive carcinomas34. The age range at diagnosis is similar to that of non-metaplastic carcinomas. Grossly the mean size is 3-4 cm, with a range of 0.5-24 cm. The tumors are firm, well delineated and solid on cut section. Microscopic examination of excised tumor is required for definitive diagnosis of metaplastic carcinoma. They are usually subdivided into two broad categoried: Carcinoma with squamous and/or spindle cell metaplasia and carcinoma with heterologous metaplasia that have a mesenchymal phenotype including chondroid or osseous metaplasia. The extent of metaplastic carcinoma can be 10-100% of the tumor35. Chronic inflammation is often present both at the periphery and with in the neoplasm. Metaplastic carcinomas are usually triple negative tumors34,35. Their prognosis is difficult to assess because of the rarity of the tumor, however majority of the studies suggest they behave as highly malignant tumors with early recurrence and poor survival34,36,37.
Tubular Carcinoma: We received one case of tubular carcinoma with mass limited to nipple and areola. The patient was a 35 years old female who had come with right breast mass in nipple and subareola, clinically diagnosed as paget’s disease of nipple. The wedge biopsy was done and reported as adenocarcinoma nipple for which mastectomy was done. Grossly the mastectomy specimen with nipple areola complex measuring 5x4x1.5 cm was received in our department. Cut section through nipple showed a greyish white growth measuring 1.5x1.1 cm limited to nipple with drainage of purulent material. Microscopy revealed it to be Tubular carcinoma of nipple (Fig 3).
Pure tubular carcinoma constitutes less than 2% of all breast carcinomas26,31,38, which is diagnosed when the tumor has clearly tubular morphology of more than 90%7,39. When the tubular component involves less than 90% of carcinoma the lesion is often referred to as “mixed tubular carcinoma or ductal carcinomas with tubular features”. Tubular carcinoma is more likely to occur in older patients, is smaller in size and have substantially less nodal involvement40 with an age range of 24 to 92 years26,38,42. Grossly most tumors are 2 cm or less in diameter but tumors as large as 4 cm have been described, they are ill defined hard masses with stellate and infiltrating cut section. They are nearly always ER, PR positive40. Pure tubular carcinoma has an excellent prognosis41,42 which in some series is similar to age matched women without breast cancer40. Microscopically they consist of simple neoplastic tubules lined by single layer of neoplastic cells. The glands can have any shape but irregular shapes and angular contours are more common.
Inflammatory carcinoma: One case of Inflammatory carcinoma was seen in our study. The patient was a 50 years old female presenting with edematous, inflammatory breast right side with multiple enlarged lymph nodes. Mastectomy with axillary lymphnode dissection was done. We received this specimen which on cut section showed a growth measuring 5x3.5x2.5 cm. Twenty-two lymphnodes were dissected out. Microscopically it was reported as Inflammatory carcinoma with metastastasis in all the lymph nodes. The clinical findings of Inflammatory mass and presence of dermal lymphatic invasion (Fig 4) fulfilled the criteria of the tumor being diagnosed as Inflammatory carcinoma and tumor was reported as pT4dN3Mx with ESBR Grade 2. According to American Joint Committee on cancer (AJCC) staging system. Inflammatory Breast Carcinoma (IBC) is defined as a clinical and pathological entity characterised by erythema and edema involving a third or more of the skin of the breast and is classified as T4d43. Inflammatory breast carcinoma accounts for 2.5% of all breast cancer cases44. The average age of IBC is 55 years. A substantial number of patients with IBC present with enlarged lymph nodes45. Our case also had clinically palpable lymph nodes which were all positive for metastasis.
Conclusion
A good variety of rare malignant epithelial tumors were seen in our study. Their correct diagnosis is important as they have peculiar prognostic and therapeutic significance. As the incidence of these rare breast cancers is same as many other studies but we would like to comment that future extensive studies and for longer time period are needed to confirm the exact incidence of these tumors.
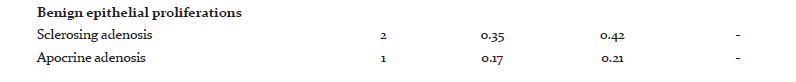
References:
1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Athers S et al: cancer incidence and mortality worldwide: sources, methods and major patterns in GOBOCAN 2012. Int J Cancer; 136, E359-E386 (2015).
2. Ghoncheh M, Momenimovahed Z, Salehiniya H. Epidemiology, incidence and mortality of breast cancer in Asia. Asian Pac J cancer Prev. 2016; 17: 47-52.
3. Malvia S, Bagdadi SA, Dubey US, Saxena S. Epidemiology of breast cancer in Indian women. Asia-Pac J Clin Oncol 2017;13: 289-295.
4. Gupta A, Shirdar k, Dhillow PK. A review of breast cancer awareness among women in India: Cancer literate or awareness deficit ? Enr J Cancer 2015; 51: 2058-66.
5. Yerushalmi R, Hayes MM, Gelmon KA. Breast carcinoma- rare types: review of literature. Ann Oncol 2009; 20: 1763-70.
6. Reimer T. Management of rare histological types of breast tumors. Breast care (Basel) 2008; 3: 190-6.
7. Ellis IO, Galea M, Broughton N et al. Pathological prognostic factors in breast cancer. Histological type: relationship with survival in a large study with long term follow up. Histopathology 1992; 20: 479-489.
8. Li CI, Uribe DJ, Darling JR. Clinical characteristics of different histological types of breast cancer. Br J Cancer 2005; 93:1046-1052.
9. Pederson L, Zedler K, Holk S, Schiodt T, Mouridsen HT (1995). Medullary carcinoma of breast, Prevalence and prognostic importance of clinical risk factors in breast cancers. Eur J Cancer 31A; 2289-2295.
10. Wargotz ES, Silverbery SG (1998). Medullary carcinoma of the breast. A clinicopathologic study with appraisal of current diagnostic criteria. Hum Pathol 1998; 19:1340-1346.
11. Jacquemier J, Reis-Filho JS, Lakhani SR, et al. Carcinoma with medullary features. In: Lakhani SR, Ellis Io, Schnitt SJ, et al; eds. WHO classification of Tumors, Vol 4, 4th edn. Lyson: World Health Organisation. IARC 2012.
12. Rakha EA, Aleskandarany M, El-Sayed ME, et al.2009. The prognostic significance of inflammation and medullary histological type in invasive carcinoma of breast. Eur J Cancer 2009;45:1780-1787.
13. Marginaean F, Rakha EA, Ho BC et al. 2010. Histological features of medullary carcinoma and prognosis in triple negative basal cell carcinomas of the breast. Mod Pathol 2010; 23:1357-1363.
14. Gamel JW, Meyer JS, Fever E, et al. The impact of stage and histology, the long term clinical course of 163,808 patients with breast carcinoma. Cancer, 1996; 77: 1459-1464.
15. Ridofi RL, Rosen PP, Port A, et al. Medullary carcinoma of the breast: a clinicopathologic study with 10 year follow up. Cancer 1977; 40: 1365-1385.
16. Fisher ER, Kenny JP, Cass R, et al. Medullary cancer of breast revisited. Breast Cancer Res Tract 1990;16:215-219.
17. Black CL, Morris DM, Goldman LI, McDonald JC. The significance of lymphnode involvement in patients with medullary carcinoma of breast. Surg Gynaecol Obstret 1983;157:497-499.
18. Rapin V, Contesso G, Mouriesse H, Bertin F, LaCombe MJ, Piekarski JD, Travalgi JP, Gadenne C, Friedmann S. Medullary breast carcinoma. A reevaluation of 95 cases of breast cancer with inflammatory stroma. Cancer 1998; 61: 2501-2510.
19. Rubens JR, Lewandrowski KB, Kopans DB, Koerner FC, Hall DA, McCarthy KA. Medullary carcinoma of breast. Overdiagnosis of a prognostically favourable neoplasm. Arch surg 1990;125: 601-604.
20. Tang F, Wei B, Tian Z et al. Invasve mammary carcinoma with neuroendocrine differentiation: histological features and diagnostic challenges. Histopathology 2011;59:106-115.
21. Righi L, Sapino A, Marchio C, et al. Neuroendocrine differentiation in breast cancers: established facts and unsolved problems. Semin Diagn Pathol 2010; 27: 69-76.
22. Zhang JY, Chen WJ. Bilateral primary breast neuroendocrine carcinoma in a young woman: report of a case. Surg Today. 2011; 41:1575-1578.
23. Kawasaki T, Mochizuhi K, Yamauchi H, et al. Neuroendocrine cells associated with neuroendocrine carcinoma of breast, nature and significance. J Clin Pathol 2012; 65: 699-703.
24. Sapino A, Papotti M, Righi L, et al. Clinical significance of neuroendocrine carcinoma of the breast. Ann Oncol 2012;12(Suppl-2): S115-117.
25. Wei B, Ding T, xing Y, et al. Invasive neuroendocrine tumors of breast: a distinctive subtype of aggressive mammary carcinoma. Cancer 2010;116:4463-4473.
26. Louwman MW, Virezen M, Van Beck MW, et al. Uncommon breast tumors in perspective: Incidence, treatment and survival in Netherlands. Int J cancer 2007;121: 127-35.
27. Scopsi L, Andreola S, Pilotti S, et al. Mucinous carcinoma of breast. A clinicopathological, histochemical and immunocytochemical study with special reference to neuroendocrine differentiation. Am J Surg Pathol 18, 702-711.
28. Rosen PP, Wang TY. Colloid carcinoma of breast. Analysis of 64 patients with long term follow up. Am J Pathol 1980;70:304.
29. Cao AY, He M, Liu ZB, et al. Outcome of pure mucinous carcinoma compared to infiltrating ductal carcinoma: a population based study from China: Ann Surg Oncol 2012; 19: 3019-3027.
30. Tokkanen and Kajuri H. Pure and mixed mucinous carcinoma of breast: a clinicopathological analysis of 61 cases along with long-term follow up. Hum Pathol 1989;20:758-764.
31. Li CI. Risk of mortality by histologic type of breast cancer in United states. Horm Cancer 2010; 758-764.
32. Komaki K, Sakamoto G, Sugano H, Morimoto T, Mondey Y. Mucinous carcinoma of the breast in Japan. A prognostic analysis based on morphologic features. Cancer 1988; 989-996.
33. Clinicopathological characteristics of Mucinous breast cancer: A retrospective analysis of a 10 year study. Lei L, Yu X, Chen B, Chen Z, Wang X (2016), PLOS ONE 11(5): e155132.
34. Khan HN, Wyld L, Dunne B et al. Spindle cell carcinoma of the breast: a case series of a rare histological type. Eur J Surg Oncol 2003; 29: 600-603.
35. Brogi E. Carcinoma with metaplasia and low grade adenocarcinoma. In: Hoda SA, Brogi E, Koener FC, Rosen PP, editors. Rosen’s Breast Pathology. 4th edn. LIPPINCOTT WILLIAMS and WILKINS, Wolter Kluwer; c-2014. p547-594.
36. Carter MR, Hornick JL, Lester S et al.2006. Spindle cell (sarcomatiod) carcinoma of breast: a clinicopathologic and immunohistochemical analysis of 29 cases. Am J Surg Pathol 30:300-309.
37. Okada N, Hasebe T, Iwasaki M, et al. 2010. Metaplastic carcinoma of the breast . Hum Pathol 2001;41:960-970.
38. Rosen PP. The pathological classification of human mammary carcinoma, Past, Present and Future. Ann Clin Lab Sci 1979; 9:144-156.
39. NHS Breast screening programme 2005. Pathology reporting of breast disease; 3rd edn. NHS Breast screening programme publication no 58. NHS cancer screening programme. Royal college of Pathologists, Sheffield, UK.
40. Diab SG, Clark GN, Osborne CK, Libby A, Allred DC, Ellede RM. Tumor characteristics and clinical outcome of tubular and mucinous breast carcinoma. J Clin Oncol 1999;17:1442-1448.
41. Peters GN, Wolff M, Hagensen CD (1981). Tubular carcinoma of the breast. Clinical pathological correlations based on 100 cases. Ann Surg 1981;193:138-149.
42. Rakh EA, Lee AH, Evan AJ et al. Tubular carcinoma of the breast, further evidence to support its excellent prognosis. J Clin Oncol 2010,28: 99-104
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License