IJCRR - 4(15), August, 2012
Pages: 06-11
Print Article
Download XML Download PDF
ANTIBACTERIAL SCREENING AND PHYTOCHEMICAL ANALYSIS OF CLEOME CILIATA (CAPPARIDACEAE) LEAVES
Author: Umerie SC., Okorie NH ., Ezea SC., Okpalaononuju A.N
Category: Healthcare
Abstract:Ethanolic extract of leaves of Cleome ciliata were studied for phytochemical constituents and in vitro antibacterial activities by agar diffusion method. The phytochemical analysis of the extract revealed the presence of saponins, alkaloids, flavonoids, tannins, steroids, terpenoids, and glycosides. The extract inhibited the growth of Staphylococcus aureus Salmonella paratyphi and Pseudomonas aeruginosa. The flavonoid fraction of the crude sample was active against Staphylococcus aureus, Escherichia coli and Klebsiella pneumoniae, while the tannin fraction was active against Staphylococcus aureus only. The minimum inhibition concentration (MIC) of the ethanolic extract as well as the flavonoid and tannin fractions ranged from 18.14 to 94.02 mg /ml. The results suggest that C. ciliata can be used in the treatment of ailments caused by the test and related organisms.
Keywords: Cleome ciliata Schmach and Thonn, antibacterial screening, phytochemical analysis.
Full Text:
INTRODUCTION
Plants have been one of the important sources of medicines since the dawn of civilization. Recently the use of plants as medicine has been patronized more vigorously, and has therefore resulted in an increase in the amount of herbal products traded within and across countries (Suresh et al, 2008). A number of well-established and important drugs have their source from plants. Plants also serve as source of chemical intermediates needed for the production of some drugs. New medicinal compounds are derived from plant species that have been used as folk, traditional or native remedies for centuries (Okafor, 2005). Information on the use of these medicinal plants has been obtained from herbalists, herb sellers, and indigenous people in Africa over many years (Sofowora, 2002). Many more of such plants are yet to come into limelight because their uses in ethno medicine have not been subjected to any scientific investigation.
Cleome ciliata Schmach and Thonn, belongs to the Capparidaceae family (Dutta, 1995) and is commonly known as ?Spider plant (USDA, 2008). The plant is a green creeping annual or short-lived perennial herb that spreads like a spider. It‘s scrambling habit smoothers and stunts young crop plant. It has a slender leaf stalk with trifoliate leaves; the leaflets are net-veined and elliptical with smooth margin. The fruit is a capsule and dry dehiscent when mature and the seeds are placed centrally axial placentation. Placement of leaves on the plant is spirally alternate and the fruits arise at the axial of the leaves. A related species Cleome rutidosperma is palatable to humans and is sometimes eaten as a cooked vegetable. Records were found of C. rutidosperma to indicate toxicity to people or animals (Jansen, 2004), but not Cleome ciliata. The plant juice in used in ethno medical practice for earache and convulsions (Oliver, 1959), and also for peptic ulcers (ASICUMPON, 2005). The present investigation was conducted to screen for the antibacterial activities and phytochemical properties of Cleome ciliata leaf extracts.
MATERIALS AND METHODS
Collection of plant material: The Cleome ciliata plant was obtained from farmlands and along roadside, in Awka Anambra State, Nigeria, and authenticated at Fame Consultancy Plant Research Centre by Prof. J.C. Okafor (consultant plant taxonomist). The leaves were air-dried and pulverized using an electric grinding machine.
Extract Preparation: The pulverized plant material (300g) was extracted by cold maceration in 1750 ml of distilled water for 24h, filtered and the filtrate concentrated over boiling water bath. A fresh batch of the plant material (600g) was extracted by cold maceration in 3 litres of absolute ethanol for 48h, filtered and the filtrate concentrated at room temperature. The exclusive extractions of flavonoids and tannins from the dried pulverized plant material were carried out by the methods of Underhill et al (1959), and Hagerman and Klucher (1986) respectively.
Phytochemical screening of crude extracts: The phytochemical components of the aqueous and ethanolic extracts of the plant were screened for using the methods of Harborne (1998) and Evans (2002). The components screened for are saponins, alkaloids, glycosides, flavonoids, terpenoids, tannins and steroids.
Preparation of extract solution: The preparation of the extract for antibacterial assay was done by the method of Okore (2005). 1.0g each of the crude ethanolic, flavonoid and tannin extracts were dissolved in separate 5ml volume of Dimethylsulphoxide (DMSO) to give a concentration of 200mg /ml. Further dilutions of the stocks were prepared to obtain desired concentrations.
Sources of micro organisms: The organisms used were clinical isolates and includes Staphylococcus aureus, Peudomonas aeruginosa, Salmonella paratyphii, Escherichia coli and Klebseilla pneumoniae. The organisms were obtained from the Department of Pharmaceutical Microbiology Laboratory, University of Nigeria Nsukka.
Standardization of bacterial suspensions: Sterile and dried nutrient agar plates were prepared and loopful from suspension of the bacteria was aseptically streaked on the surface of solid agar medium in different Petri dishes and the dishes were incubated at 37OC for 24 hours. A colony was aseptically removed from each of the growths and inoculated into preparation double strength nutrient broth in bijou bottle. After incubation, the contents of each bottle were used to flood the surface of the solid nutrient agar slant in roux bottles. They were then incubated for 24 hours. The surface growths on the agar slant were washed with sterile normal saline by centrifugation. The microorganism were bulked in sterile bottles and made up to 10ml with sterile normal saline. Further dilutions with sterile normal saline were made using McFarland standard to obtain the required concentration of approximately 107 Cfu/ml and the organisms were stored in the refrigerator at 4OC until when required for use (Okore, 2005; Anidu, 2002).
Antibacterial screening: The antibacterial activities of the extracts measured as inhibition zone diameter (IZD) was determined using agar diffusion (cup plate) method. The agar plates were incubated at 37OC for 24 hours after which the IZD values were obtained (Okore, 2005). The experiment was replicated and the mean IZD was calculated. The minimum inhibitory concentration (MIC) value was then obtained from the intercept on the natural Iogarithm of extract concentration axis of a graph of natural logarithm of extract concentration against squared IZD (mm2 ). The graph was plotted for each bacterial and their MIC values obtained.
RESULTS
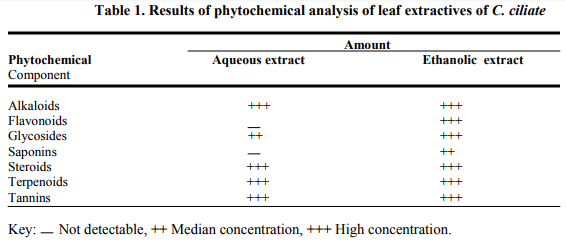
Phytochemical Screening: Phytochemical screening of the ethanolic extract of C. ciliata indicated the presence of alkaloids, flavonoids, glycosides, saponins, tannins, steroids, and terpenoids. However, flavonoids and saponins were not detected in the aqueous extract (Table 1). The yield of the extract was 10.6%
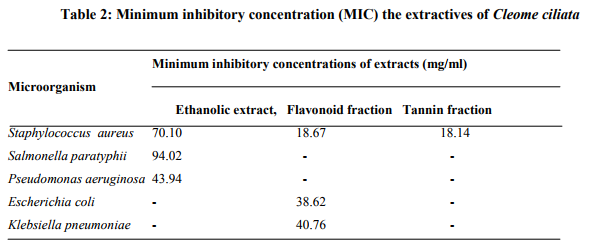
Antibacterial activity of extracts: The results in Table 2 revealed that the ethanolic extract exhibited antibacterial effects on Staph. aureus, Sal. Paratyphis and P. aeruginosa but not on E. coli and K. pneumoniae, The flavonoid extract was active against Staph aureus, E. coil and K. preumoniae only, while the tannin extract was active only against Staph. aureas.
Minimum inhibition concentration (MIC) of the extracts: The MIC of the ethanolic extract for Staph.aureus was 70.10 mg/ml, 94.02 mg/ml for Sal. paratyphii and 43.94 mg/ml for P. aeruginosa. The MIC of the flavonoid extract was 18.67mg/ml against Staph aureus, 38.26mg/ml against E. coil and 40.76 mg/ml against K. pneumoniae while the MIC of tannin extract was 18.14 mg/ml against Staph. aureus. (Table 3).
DISCUSSION
The yield of the ethanolic extract of C. ciliate revealed that their extractive yield is high in polar solvents. This implies that phytochemical components of the plant are polar in nature and substantiates the use of water or ethanol (local gin) as extracting solvents in folkloric medicine. Moreso these classes of components are known to show curative activity against pathogens (Usman and Osuji, 2007).
The crude ethanolic extract was more active in inhibiting Staphylococus aureus, followed by Salmonella paratyphii and Pseudomonas aeruginosa than the tannin and flavonoid fractions. The flavonoid fraction had a wider range of activity than the tannin fraction. The activity of the flavonoid fraction against E. coli and Klebsiella spp. relative to the crude extract suggested that certain phytocomponents could have hindered its activity in the crude sample. This may be associated with the presence of oils, wax, resin, fatty acids or pigments, which has been reported to be capable of blacking the active ingredients in the plant extract, thus preventing the plant extract from accessing the plant cell wall (Doughari and Manzara, 2008).
Flavonoids are hydroxylated phenols and are toxic to microorganisms. Their relative activities increase with increasing level of oxidation (Scalbert, 1991), number of hydroxyl groups and their specific sites (Geissman, 1963) They and the phenols have the ability to complex with nucleophilic amino acids in proteins and bacterial cell walls leading to enzyme inactivation and loss of function (Ogunwenmo et al, 2007).
Water-soluble polyphenols, tannins are toxic to filmentous fungi, yeasts and bacteria (Scalbert, 1991). They owe their antibacterial action to their capacity for protein complexation through hydrogen and covalent bonding and inactivation of microbial adhesions, enzymes and cell envelope transport proteins (Haslam, 1996, Stern et al, 1996). Condensed tannins are known to bind to cell wall of ruminal bacteria preventing growth and protease activity. Hence the consumption of tannins as green teas and wine prevents different illnesses (Ogunwenmo et al, 2007). Consequently consumption of tannins as herbal vegetable like C. ciliata will have the same effect.
Staphylococcus aureus showed highest level of susceptibility than other microorganisms tested and it was sensitive on the three extracts used. This suggested that crude, flavonoid and tannin extracts of C. ciliata could be used in treatment of disease involving this particular bacterium such as in cases of gastrointestinal disorders (Aguwa and Ukwe 1997; Dalziel, 1985). The minimum inhibitory concentration (MIC) values showed that Staph, aureus had the lowest MIC for all the extracts and so very low doses of the extracts would be required to inhibit the growth of the organism Staph. aureus. Very high doses will be required to inhibit the growth of Klebsiella, E.coli Salmonella paralyphi and Pseudomonas aeruginosa.
In conclusion, the extracts of C. ciliata have antibacterial properties. The MIC investigation showed that crude extract flavonoid and tannin fractions were able to inhibit microorganism. The study has therefore justified the use of the plant in ethno medicine.
References:
1. Aguwa, C.N. and Ukwe, C.V. (1997). Gastrointestinal activities of Sterculla tragacantha leaf extract. Fitoterapia 68 (2): 127-132.
2. Anidu, U.J. (2002). An investigation into the antimicrobial properties of the oil extracts of seeds of Picralima nitida. B. Pharm project, Department of Pharmacognosy, University of Nigeria, Nsukka. pp 1-20.
3. ASICUMPON, (2005). The Association for Scientific Identification, Conservation and Utilization of Medicinal Plants of Nigeria Checklist of Medicinal Plants of Nigeria and their uses. Trinity – Biz Publishers, AbakpaEnugu.
4. Dalziel, J.M. (1985). Useful Plants of West Tropical Africa. Crown Agents for Overseas Government and Administration, London. pp 109-110.
5. Doughari, J.H. and Manzara, S. (2008) In vitro antibacterial activity of crude leaf extracts of Magnifera indica Linn. African Journal of Microbiology Research 2: 67-72.
6. Dutta, A.C. (1995) Bontany Degree Students, 6 th edition. Oxford University Press, Calcutta, India.
7. Evans, W.C. (2002). Trease and Evans Pharmacognosy, 15th edition, W.B. Saunders Company Limited, Edinburgh, UK.
8. Geissman, T.A. (1963). Flavonoid compounds, tannins, lignins and related compounds. In: Florkin, M and Stotz, E.Z. (ed). Pyrrole pigments, isoprenoid compounds and phenolic plant constituents, Vol. 9, Elsevier, New York.
9. Hagerman, A.E. and Klucher, K.M. (1986). Tannin-protein interaction. In: Plant Flavonoids in Biology and Medicine: biochemical, pharmacological, and structure activity relationship, New York,
10. Harborne, J.B. (1998). Phytochemical methods: A guide to modern techniques of plant analysis, 3rd edition. Chapman and Hall, London, UK.
11. Haslam, E (1996). Natural polyphenols (vegetable tannins) as drugs: possible mode of action. J. Nat. Prod. 59: 205-215.
12. Jansen, P.C.M. (2004). Cleome rutidosperma http// database. Prota.org/search (14/04/2008). Ogunwenmo, K.O., Idowu, O.A., Chukwudi, I. Esan, E.B. and Oyelane
13. O.A. (2007). Cultivers of Codiaeum variegatum (L) Blume (Euphorbiaceae) show variability in phytochemical and cytological characteristics. African Journal of Biotechnology 6 (20): 2400-2405.
14. Okafor, J.C. (2005). Strategies for conservation of the genetic resources of medicinal plants of Nigeria. In: Checklist of Medicinal Plants of Nigeria and their uses. Trinity-Biz Publishers, Abakpa-Enugu pp 9- 12.
15. Okore, V.C. (2005), Pharmaceutical Microbiology, 1st edition. ELDMAK Publishing Co. Ltd., Enugu, Nigeria
16. Oliver, E.W.H.M. (1959). Medicinal plants of Nigeria, Part II. Technical Memorandum No. 7, Federal Institute of Industrial Research. Published by the Federal Ministry of Commerce and Industry, Lagos, Nigeria.
17. Scalbert, A (1991). Antimicrobial properties of tannins. Phytochemistry 30:3875-3883.
18. Sofowora, A. (2002). Plants in African traditional Medicine-an overview. In: Trease and Evans Pharmacognosy 15th edition, by W.C. Evans. W. Saunder Company Limited, Edinburgh, Uk. pp 488-496.
19. Stern, J.L., Hagerman, A.E., Steinberg, P.D. and Mason, P.K. (1996). Phlorotannin-protein interactions. J. Chem. Ecol. 22:1887-1899. 10 International Journal of Current Research and Review www.ijcrr.com Vol. 04 issue 15 Aug 2012
20. Suresh, K., Deepa, P., Harisaranraji, R. and Vaira Achudhan (2008). Antimicrobial and Phytochemical investigation of the Leaves of Carica papaya L., Cynodon dactylon (L) Pers., Euphorbia hirta L., Melia azedarach L. and Psidium guajava L. Ethnobotanical Leaflets 12: 1184-1191.
21. Underhill E.W., Walkin J.E. and Neish, A.C. (1957) Flavonoids. Canadian J. Biochem. Physiol. 35:219-237.
22. USDA,ARS (2008). National Genetic Resource Program Germplasm Resources Information Network – (GRIN) [Online Natabase]. National Germplasm Resources Laboratory, Beltsville, Maryland. URL:http//.www.ars-grin. Gov/cgibin/html/tax-search pl (12/04/2008).
23. Usman, H. and Osuji, J.C. (2007). Phytochemical and in vitro antimicrobial assay of the leaf extract of Newbouldia laevis. African Journal of Traditional, Complementary and Alternative Medicine 4 (4):476-480.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License