IJCRR - 9(20), October, 2017
Pages: 24-29
Print Article
Download XML Download PDF
Histopathological Study of Ocular Surface Squamous Neoplasia in Biopsies Received at Tertiary Care Centre
Author: Neetal Desai, Ami Shah, Hansa Goswami
Category: Healthcare
Abstract:Introduction: Ocular surface squamous neoplasia(OSSN) is an encompassing term for pre-cancerous and cancerous epithelial lesions of the conjunctiva and cornea which includes Dysplasia, Carcinoma in-situ (CIS) and Invasive Squamous Cell Carcinoma. OSSN is mostly unilateral, occurs in middle aged to older patients. It is associated with factors such as HIV and xeroderma pigmentosum.
Aims and Objectives:
1) To evaluate incidence of Dysplasia and Squamous Cell Carcinoma in biopsies received
2) To study the spectrum of histopathological findings in Ocular Surface Squamous Neoplasia
Materials and Method: We examined 52 ocular surface biopsies received in our tertiary care centre during the period of 22 months (August-2015 to May-2017). Slides stained with Hematoxylin and Eosin were examined and dysplasia found in biopsies were categorized into mild, moderate and severe. Invasive squamous cell carcinomas were again categorized according to differentiation into well differentiated, moderately differentiated and poorly differentiated. In addition to these 52 biopsies, 10 biopsies were excluded from the study which showed normal or only hyperplastic squamous epithelium.
Results: Out of 52 biopsies examined, 2 cases showed squamous papilloma,13 cases showed dysplasia-mild being the commonest and 37 cases showed invasive carcinoma, moderate differentiation being the commonest.
Conclusion: OSSN is common in middle age and male gender and usually occurs in limbal conjunctiva. Histopathology remains the gold standard for accurate diagnosis and grading of OSSN. Despite increasing awareness, Invasive carcinoma is more prevalent than dysplasia. Prognosis largely depends upon grade and differentiation of tumor and specific microscopic type.
Keywords: Dysplasia, Squamous Cell Carcinoma, HIV, Xeroderma Pigmentosum
Full Text:
Introduction:
Ocular Surface Squamous Neoplasia(OSSN) is an embracing term for pre-cancerous and cancerous epithelial lesions of the conjunctiva and cornea. It includes the spectrum of Dysplasia, Carcinoma in-situ (CIS) and Invasive Squamous Cell Carcinoma(SCC)(1-3).
OSSN is mostly unilateral and is seen in middle age and older patients. Rarely, it is bilateral especially in immunocompromised patients. Factors causing development of OSSN are exposure to sunlight, HPV type 16 infections and HIV infection(2,5). There is a systemic association of xeroderma pigmentosum with OSSN patients. Other factors associated are old age, heavy cigarette smoking, male sex and light complexion.
Clinically, we cannot distinguish CIS from invasive SCC. The presence of feeder vessels, intrinsic vascularity and a nodular lesion should raise suspicion of invasive SCC. This usually presents either as a fleshy, gelatinous lesion or as a sessile, papillomatous lesion mostly in the interpalpebral region. Unless the lesion is encroaching onto the pupillary area, vision is spared. Presenting clinical features of this entity are swelling, redness, irritation and feeder vessels surrounding the lesion (1,2,5,7,8).
Advanced cases can invade the cornea and sclera (9) and on rare occasions the tumor may infiltrate into the orbit causing proptosis.
Treatment includes complete surgical excision with 4mm margin clearance without touching the tumor, so called 'No Touch Technique'(1,10,11). Cryotherapy is also useful (8)
Reported recurrence rate of OSSN is 15-52%. Lee and Herst reported a 17% recurrence after excision of conjunctival dysplasia, 40% after excision of CIS and 30% for SCC of conjunctiva2. The recurrence rate can be limited to less than 5% with the above technique. OSSN has a good prognosis. The recurrence rate is about 5% and regional metastasis rate is about 2% With the modern techniques(1,5,12,13) .
Aims and objectives:
1)To evaluate incidence of Dysplasia and Squamous Cell Carcinoma in biopsies received
2)To study the spectrum of histopathological findings in Ocular Surface Squamous Neoplasia
Materials and method:
We examined 52 ocular surface biopsies received in our tertiary care centre during the period of 22 months(August-2015 to May-2017).Slides were stained with Hematoxylin and Eosin. Dysplasia found in biopsies were categorized into mild, moderate and severe (Conjunctival Intraepithelial Neoplasia(CIN)-I,II and III respectively). Slides were carefully examined for foci of invasion. Invasive squamous cell carcinomas were again categorized according to differentiation into well differentiated, moderately differentiated and poorly differentiated. In addition to these 52 biopsies, 10 biopsies were excluded from the study which showed normal or only hyperplastic squamous epithelium.
Results:
Out of 52 biopsies examined, results are as shown in (Table:1), with comparison with the study done in 2016 by Kabra RC et al(14).(Table: 2)
Two cases of poorly differentiated carcinoma showed histomorphology of spindle cell variant-a known variant of invasive ocular squamous cell carcinoma. One of them was HIV positive patient. In the study by Kabra RC et al, more patients were HIV positive(14).
A few cases with moderately and poorly differentiated carcinoma showed extensive stromal necrosis.
Maximum(42) cases were found between the age group of 41-80 years, mean age being 57.5 years. A few cases of moderate dysplasia and well differentiated carcinoma were found in the younger age group(<20 years of age).One male patient was 26 years of age and was a known case of xeroderma pigmentosum, showed histology of well differentiated squamous cell carcinoma on biopsy. Out of 52 cases, 35 were male and 17 were female. Results are compared with the study done by Kabra RC et al(14), in which mean age was 38.2 years and males were more commonly affected(14).
Most common location was limbal conjunctiva,30 out of 52 cases.Other locations are nasal and temporal conjunctiva.
Discussion:
Ocular Surface Squamous Neoplasia, within its spectrum, includes Squamous papilloma, Intraepithelial neoplasia or Dysplasia, Intraepithelial Carcinoma and Squamous Cell Carcinoma. The term CIN(Conjunctival intraepithelial neoplasia)-which includes dysplasia and carcinoma in-situ, in vogue today was proposed by Pizarello and Jakobeic, derived from the terminology applied to the intraepithelial cervical malignancies(15).
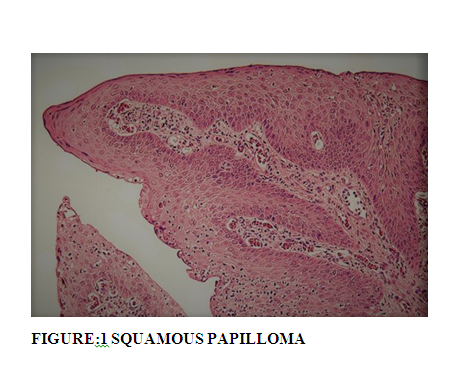
Squamous papilloma of conjunctiva lack malignant potential and when occur in children, they are mostly bilateral and recurrent and associated with HPV infection. When in adults, they are usually solitary and unilateral and confused clinically with squamous cell carcinoma (16).In our study both the cases of squamous papilloma occurred in elder age group with solitary and unilateral lesions.
Histopathologically, both cases of papilloma showed papillomatous fronds of squamous epithelium that covered a fibrovascular core. (Figure: 1) It is reported that about 6% of squamous papillomas can present epithelial dysplasia(17).Both cases did not show any changes of dysplasia in our study.
Abnormalities in a dysplastic lesion is because of the deranged cellular proliferation that means the abnormal mitotic cells do not fully differentiate as they move upwards in the epithelium (disordered proliferation or altered differentiation).
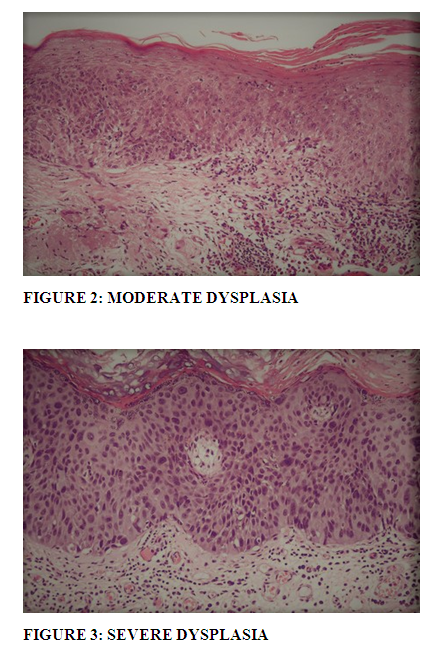
Dysplasia of the conjunctival epithelium is characterized by acanthosis, loss of cellular polarity, and cellular pleomorphism, and it resembles dysplasia of the uterine cervix microscopically. Depending on the extent of the epithelial abnormalities, conjunctival dysplasia can be designated as mild, moderate, or severe(16).(Table: 3).
Full thickness epithelial dysplasia without breaching of epithelial basement membrane, is known as carcinoma in situ. As explained by Mittal R et al (18), as the lesion progresses from mild to severe dysplasia, the cells differentiate less and less, losing their squamous features until eventually entire epithelium consists of undifferentiated cells. The dysplastic cells show an increase in the size of nuclei, irregular nuclear membrane, hyperchromatic and fine to coarse nuclear chromatin. Degree of nuclear atypia, irrespective of level of epithelial thickness involved can also cause up gradation or down gradation of severity of dysplasia. Mittal R et al (18) also explained in their study that hyperkeratosis and dyskeratosis that is single cell keratinization with the formation of squamous pearls is not a usual feature of intra epithelial lesions. Increased mitosis with presence of abnormal mitosis can be observed. Mild to moderate chronic inflammatory cell infiltration may be observed and can cause inflammatory destruction of epithelial basement membrane. This should not be confused with invasive squamous cell carcinoma, especially in cases of carcinoma in situ as (18).
Nuclear atypia was found in all 9 cases of dysplasia, though more pronounced in moderate and severe dysplasia.(Figure: 2 and 3) Some cases of mild dysplasia showed hyperkeratosis but dyskeratosis and pearl formation were not found in any of the cases. Frequent mitosis with atypical mitosis were observed.
Lesions associated with HPV show cytopathic effect in cells, resulting in the formation of a halo around the enlarged and wrinkled nucleus with dense chromatin and inconspicuous nucleoli. The halo is surrounded by dense eosinophilic cytoplasm. Such cells are called koilocytes. Koilocytes are pathognomic of HPV infection. As explained by Mittal R et al (18), formation of koilocytes decreases with increasing severity of dysplasia, and they are rarely seen in invasive lesions(18). In our study, none of the cases showed this feature.
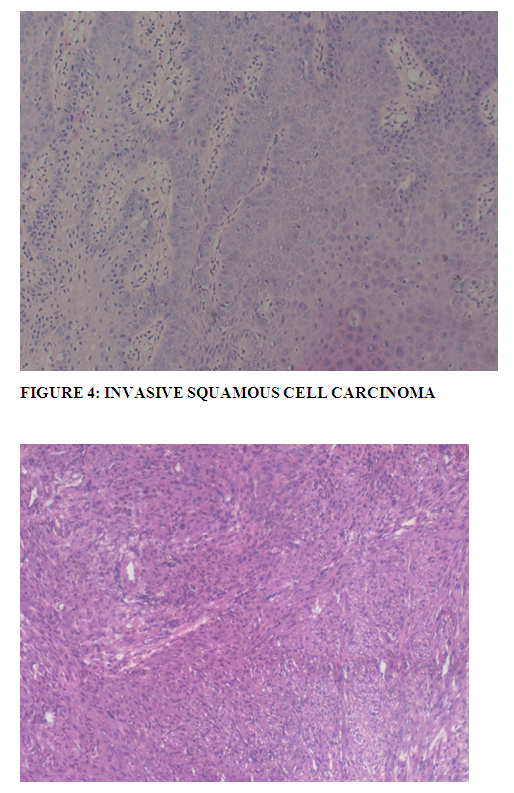
Invasive squamous cell carcinoma develops from conjunctival intraepithelial neoplasia by breaching the epithelial basement membrane and invading the underlying stromal tissue. As mentioned by Mittal R et al (18), invading tumor can have several patterns like cords, strands and clusters of malignant cells in infiltrative manner, broad pushing fronds with expansive pattern can also be seen. Dense collagenous scleral tissue in cases of conjunctival OSSN and Bowman's membrane in corneal OSSN helps limiting the infiltration. Cytologically cells are large polygonal, but can be small to spindle in shape. Authors also mention that the more the degree of maturation, the dense, eosinophilic is the cytoplasm, the pyknotic is the nucleus. There is single cell keratinization or formation of squamous pearl. Differentiation is in terms of degree of keratinization and accordingly named as well-differentiated, moderately differentiated and poorly differentiated carcinoma. As observed by Mittal R et al (18) in their study, keratinization is more in well and moderately differentiated tumors with less nuclear pleomorphism cytologically, whereas poorly differentiated tumors have predominance of immature cells with higher nuclear-cytoplasmic ratio, more pleomorphic nuclei, increased typical and atypical mitoses and minimal keratinization.(18).
Majority (20) cases showed moderately differentiated squamous cell carcinoma with moderate keratinization with polygonal cells with moderate amount of cytoplasm, enlarged nuclei and mild nuclear atypia with frequent mitosis.(Figure: 4) One case showed extensive stromal necrosis. Few cases showing well differentiation were difficult to distinguish from carcinoma in-situ especially in cases which showed only occasional foci of microinvasion-termed as microinvasive squamous cell carcinoma. Vascular invasion was also seen in some cases. Out of 17 well differentiated SCC,5 cases showed microinvasion.
All 4 cases of poorly differentiated tumors showed features mentioned above.Cells showed increased nuclear atypia and mitosis than moderately or well differentiated cases.
Invasive squamous cell carcinoma needs to be differentiated from pseudoepitheliomatous hyperplasia. Pseudoepitheliomatous hyperplasia is a reactive proliferation of the surface epithelium secondary to an inflammatory or infectious process. Histologically it is confused with well differentiated or moderately differentiated carcinoma because it shows irregular invasion, keratin pearl formation and mitotic figures. As mentioned by Mittal R et al (18), disparity between architectural and cytological abnormality with minimal or absent nuclear hyperchromasia, hyperplasia and pleomorphism, helps in differentiating from a true malignancy(18,19).
Another differential diagnosis is keratoacanthoma. Microscopically,there are lobules of proliferating squamous epithelium which grows upward and inward and may have increased number of mitotic figures with vesicular nuclei and eosinophilic nucleoli raising the suspicion of SCC.In such cases it should be indicated in the pathology report that SCC cannot be definitively excluded and only the biologic behavior of the lesion will differentiate both the lesions(19).
Other ocular surface lesions which can clinically mimic OSSN such as actinic keratosis, pterygium, pinguecula and actinic granuloma can be easily differentiated from OSSN microscopically (18). Pigmented SCC can be confused with melanoma, IHC for keratin establishes the diagnosis(19).
Clear cell change/hydropic change of the cytoplasm can be seen in squamous cell carcinoma and one should be cautious while dealing with it because it mimics an aggressive disease such as pagetoid extension in a sebaceous gland carcinoma, Mucoepidermoid carcinoma, or even metastatic renal cell carcinoma, as explained by Mittal R et al (18).
There are variants of Invasive Squamous cell carcinoma such as Mucoepidermoid carcinoma, Spindle cell carcinoma, papillary squamous cell carcinoma and Acantholytic/Adenoid squamous cell carcinoma(18).
Mucoepidermoid carcinoma is a locally aggressive, mucin-producing variant of squamous cell carcinoma with same clinical presentation as classic squamous cell carcinoma. Histopathology is required to make this rare diagnosis. Microscopic features are well documented by Mittal R et al (18) in their study, which include admixture of epidermoid cells, intermediate cells,and mucus producing cells (components can vary within the same tumor), as well as sheets or cords of squamous cells with eosinophilic cytoplasm and predominantly mucin-containing cells with pools of mucin surrounded by the neoplastic cells. Mittal R et al (18) also explains that the presence of squamous pearls is the exception rather than the rule. Mucoepidermoid carcinoma has a high rate of recurrence, but local and distant metastases are uncommon.(18,19).
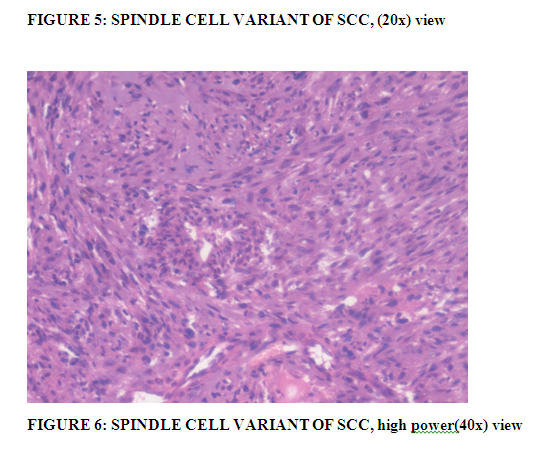
Spindle cell carcinoma is another rare and locally more aggressive variant of squamous cell carcinoma of conjunctiva with only few case reports in the literature. Spindle cell carcinoma/Sarcomatoid carcinoma microscopically shows biphasic pattern of squamous cell carcinoma with a larger component of malignant spindle cells. The squamous component can be scanty or even not at all apparent on light microscopy (Figure: 5 and 6). In such cases, immunohistochemical stains for cytokeratins, vimentin, p63 and EMA and electron microscopic evidence of squamous differentiation is required for diagnosis as explained by Mittal R et al (18). Spindle cell carcinoma is much more likely to extend through the sclera, cornea and the interior of the globe(18).
Two such cases were observed in our study showing poorly differentiated squamous cell carcinoma with spindle cell morphology, one of them was an HIV reactive patient.
Papillary squamous cell carcinoma is an exophytic variant of squamous cell carcinoma with a papillary configuration that is tumor cells arranged in papillary fashion surrounding fibrovascular core. They generally have a better prognosis(18).
Adenoid squamous cell carcinoma or Acantholytic squamous cell carcinoma is another rare, locally aggressive and recurrent variant of SCC. It can even metastasize and should be microscopically differentiated from the less aggressive conventional squamous cell carcinoma. Regular follow up is needed to predict recurrences (18).
Management modalities for OSSN include complete surgical excision in well delineated tumors, wide excision in aggressive SCC variants like mucoepidermoid and spindle cell SCC to topical chemotherapy(5-fluorouracil and mitomycin C) and immunotherapy in diffuse unresectable lesions.
The overall prognosis in OSSN is good. Mittal R et al (18) mentioned in their study that modern treatment strategies are effective with lower local recurrence rates and regional lymph node metastasis. Aggressive variants like muco-epidermoid and spindle cell carcinoma and OSSN in immunocompromised patients have a worse prognosis(18).
Conclusion:
OSSN is common in middle age and male gender and has an association with HPV as well as HIV infections and xeroderma pigmentosum. Histopathology remains the gold standard for accurate diagnosis and grading of OSSN. Despite increasing awareness, Invasive carcinoma is more prevalent than dysplasia. Prognosis largely depends upon grade and differentiation of tumor as well as specific microscopic types.HIV status also predicts prognosis.
Acknowledgement:
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors/editors/publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
Note: The present study was undertaken at tertiary care centre, Gujarat and all the biopsies considered in the present study were taken at this centre with prior consent of the patients. Ethical committee clearance has not been required as confidentiality of patient's details has not been published.
TABLE: 1 Number of cases of dysplasia,carcinoma in-situ and invasive carcinoma in different age groups.
|
|
Age
|
|
*OSSN-lesion
|
0-20
|
21-40
|
41-60
|
61-80
|
Total
|
|
Papilloma
|
0
|
0
|
1
|
1
|
2
|
|
Intraepithelial neoplasia-Dysplasia
|
|
|
|
|
|
|
Mild
|
0
|
0
|
5
|
1
|
6
|
|
Moderate
|
1
|
0
|
1
|
0
|
2
|
|
Severe
|
0
|
0
|
0
|
1
|
1
|
|
Carcinoma In-Situ
|
0
|
0
|
0
|
0
|
0
|
|
Invasive Carcinoma
|
|
|
|
|
|
|
Well differentiated
|
1
|
3
|
4
|
4
|
12
|
|
Microinvasive carcinoma
|
0
|
1
|
3
|
1
|
5
|
|
Moderately differentiated
|
0
|
4
|
13
|
3
|
20
|
|
Poorly differentiated
|
0
|
0
|
3
|
1
|
4
|
|
TOTAL
|
52
|
(*OSSN- Ocular Surface Squamous Neoplasia)
TABLE:2 Comparison between current study and study by Kabra RC et al(14).
|
*OSSN-lesion
|
Current Study
|
Study by Kabra RC et al(14)
|
|
Papilloma
|
2
|
-
|
|
Intraepithelial neoplasia-Dysplasia
|
|
|
|
Mild
|
6
|
12
|
|
Moderate
|
2
|
2
|
|
Severe
|
1
|
9
|
|
Carcinoma In-Situ
|
0
|
-
|
|
Invasive Carcinoma
|
|
|
|
Well differentiated
|
12
|
9
|
|
Microinvasive carcinoma
|
5
|
-
|
|
Moderately differentiated
|
20
|
9
|
|
Poorly differentiated
|
4
|
14
|
(*OSSN- Ocular Surface Squamous Neoplasia)
TABLE: 3 Grading of dysplasia
|
Degree of Dysplasia
|
Microscopic Finding
|
|
Mild (*CIN I)
|
Lower 1/3rd of the epithelium shows dysplasia
|
|
Moderate (*CIN II)
|
Lower 2/3rd of the epithelium shows dysplasia
|
|
Severe (*CIN III)
|
>2/3rd of the epithelium shows dysplasia, however surface maturation is preserved.
|
(*CIN- Conjunctival Intraepithelial Neoplasia)
References:
References:
(1) Shields CL, Shields JA. Tumours of the conjunctiva and cornea. Surv Ophthalmol. 2004;49:3-24.[PubMed]
(2) Lee GA, Hirst LW. Ocular surface squamous neoplasia. Surv Ophthalmol. 1995;39:429-50. [PubMed]
(3) Pe'er J. Ocular surface squamous neoplasia. Ophthalmol Clin North Am. 2005;18:1-13. vii. [PubMed]
(4) Lee GA, Hirst LW. Retrospective study of OSSN. Aust NZJ Ophthalmol. 1997;25:269. [PubMed]
(5) Shields JA, Shields CL. Eyelid, Conjunctival and Orbital Tumours. An Atlas and Textbook. 2nd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2008. pp. 250-445.
(6) Shields CL, Demirci H, Karatza E, Shields JA. Clinical survey of 1643 melanocytic and nonmelanocytic conjunctival tumours. Ophthalmology. 2004;111:1747-54. [PubMed]
(7) Farah S, Baum TD, Conton MR. Principles and Practice of Ophthalmology. 2nd ed. Philadelphia: WB Saunders; 2000. Tumours of cornea and conjunctiva. In: Albert DM, Jakobiec FA, editors; pp. 1002-19.
(8) Honavar SG, Manjandavida FP. Tumours of the ocular surface: A review. Indian J Ophthalmol. 2015;63:187-203. [PMC free article] [PubMed]
(9) Nicholson DH, Herschler J. Intraocular extension of squamous cell carcinoma of the conjunctiva. Arch Ophthalmol. 1977;95:843-46. [PubMed]
(10) Mauriello JA, Napolitano J, McLean I. Actinic keratosis and dysplasia of the conjunctiva: A clinicopathological study of 45 cases. Can J Ophthalmol. 1995;30:312-16. [PubMed]
(11) Shields JA, Shields CL, De Potter P. Surgical management of conjunctival tumours. The 1994 Lynn B. McMahan Lecture. Arch Ophthalmol. 1997;115:808-15. [PubMed]
(12) Cervantes G, Rodríguez AA, Leal AG. Squamous cell carcinoma of the conjunctiva: Clinicopathological features in 287 cases. Can J Ophthalmol. 2002;37:14-19. [PubMed]
(13) Shields CL, Fasiuddin AF, Mashayekhi A, Shields JA. Conjunctival nevi: Clinical features and natural course in 410 consecutive patients. Arch Ophthalmol. 2004;122:167-75. [PubMed]
(14)Kabra RC, Morawala A, Maheshwari VN. Clinicopathological analysis of 55 cases of ocular surface squamous neoplasia. Ophthal Rev: Int J ophtha and Oto. 2016;1(1):10-16. doi: 10.17511/jooo.2016.i1.04.
(15)Pizzarello L.D., Jakobiec F.A. Bowen's disease of the conjunctiva: a misomer. In: Jakobiec F.A., editor. Ocular adnexal tumors. Aesculapius; Birmingham, AL: 1978. pp. 553-571.
(16) Alexandre N. Odashiro, Thomas J. Cummings, Miguel N. Burnier, Jr. Eye and Ocular Adnexa. In: Stacey E. Mills, editor; Joel K. Greenson, Jason L. Hornick, Teri A. Longacre, Victor E. Reuter, Associate editors.Sternberg's diagnostic surgical pathology. 6th ed. Philadelphia: Wolters Kluwer Health; 2015. p. 1074-1075.
(17)Sjö N, Heegaard S, Prause JU. Conjunctival papilloma.A histopathologically based retrospective study.Acta Ophthalmol Scand 2000;78:663-666.
(18)Mittal R, Rath S, Vemuganti GK. Ocular surface squamous neoplasia - Review of etio-pathogenesis and an update on clinico-pathological diagnosis. Saudi Journal of Ophthalmology. 2013;27(3):177-186. doi:10.1016/j.sjopt.2013.07.002.
(19)Ramon L Font, J Oscar Croxatto, Narsing A Rao.Tumors of the Eye and Ocular Adnexa, AFIP Atlas of Tumor Pathology. Series 4, fascicle 5. The American Registry of Pathology in collaboration with the Armed Forces Institute of Pathology; 2006. p. 1-10.










 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License