IJCRR - 9(17), September, 2017
Pages: 26-31
Print Article
Download XML Download PDF
The Configuration and Variations in the Developing Circle of Willis- A Retrospective Autopsy Study
Author: Kanchan Kapoor, Jyotsna Singh, Joseph Abraham, Amrutha Kavil Valapil
Category: Healthcare
Abstract:Introduction: Cerebral hemodynamics undergoes a shift from carotid system to vertebro-basilar system from fetal stage to adult life. The circle of Willis (CW) plays a major role in redistributing the blood and enables interhemispheric flow through the anterior and posterior communicating arteries (AComA, PComA). The text book description mention three stages in the development of CW- fetal, transitional and adult. The purpose of the present study was to observe development of arterial anastomoses during intrauterine life, to note the variations and trying to explain some of the hypothesis for these variations.
Material and Method: The material of the present study include 45 fetal brains of different age groups. Variations were observed regarding absence, duplication, bilateral asymmetry and tortuousity.
Results: The number and type of variations in fetus were lesser as compared to adults. Anomalies including presence of aneurysm, absence of anterior cerebral artery (ACA) and splitting of a constituent vessel were not observed in fetal brains. However there were incidences of absence / duplication and hypoplasia of a component vessel. During early gestation period, all the cerebral vessels were slender but uniform in size. This is named as fetal stage. After 22nd wks of fertilization, due to rapid development of occipital lobes, there is increase in the diameter of either posterior cerebral artery (PCA) or PComA thereby giving rise to either adult or transitional type of configuration respectively.
Keywords: Fetal brains, Circle of Willis, Variations
DOI: 10.7324/IJCRR.2017.9174
Full Text:
INTRODUCTION
The circle of Willis (CW) is formed at the termination of two internal carotid arteries and joining of vertebrobasilar trunk with its branches. All arteries of the forebrain arise from this anastomotic polygon. The circle of Willis (CW) plays a major role in redistributing the blood and enables inter-hemispheric flow through the anterior and posterior communicating arteries. The normal configuration of circle of Willis (CW) appears in a 52nd day embryo and its variations are well described in literature1. Although many cerebral arterial variants may be asymptomatic, their recognition is important in patients with cerebrovascular disease and in those who undergo brain imaging for other purposes, as some of these variants might be pathological or turn pathological in a surgical setting. Fenestrations and/or duplication of intracranial arteries have been associated with the development of brain saccular aneurysm 2.The present study is an attempt to observe the development of arterial anastomosis during intrauterine life and to note the variations. An effort has been made to explain the hypothesis for the occurrence of these variations.
MATERIAL AND METHODS
The material for the present study included 45 fetal brains in 14-30 weeks of gestation. The fetal brains were carefully removed and examined with an dissecting microscope during routine autopsy performed at Department of Anatomy, Government Medical College and Hospital, Chandigarh,
The circle of Willis (CW) is formed at the termination of two internal carotid arteries and joining of vertebrobasilar trunk with its branches. All arteries of the forebrain arise from this anastomotic polygon. The circle of Willis (CW) plays a major role in redistributing the blood and enables inter-hemispheric flow through the anterior and posterior communicating arteries. The normal configuration of circle of Willis (CW) appears in a 52nd day embryo and its variations are well described in literature1. Although many cerebral arterial variants may be asymptomatic, their recognition is important in patients with cerebrovascular disease and in those who undergo brain imaging for other purposes, as some of these variants might be pathological or turn pathological in a surgical setting. Fenestrations and/or duplication of intracranial arteries have been associated with the development of brain saccular aneurysm 2.The present study is an attempt to observe the development of arterial anastomosis during intrauterine life and to note the variations. An effort has been made to explain the hypothesis for the occurrence of these variations.
Material and methods
The material for the present study included 45 fetal brains in 14-30 weeks of gestation. The fetal brains were carefully removed and examined with an dissecting microscope during routine autopsy performed at Department of Anatomy, Government Medical College and Hospital, Chandigarh, India. The approval from the ethical committee of the institute was taken prior to the autopsy. The fetuses which were sent for autopsy were obtained from spontaneous or clinical abortions in the Obstetrics and Gynaecology department of the institute. Prior to the autopsy consent was taken from the parents. Cases with gross pathological lesions of the brain were excluded. After removal of brains, the cerebral vessels were examined at the base of the brain. Variations were observed in the cases regarding absence, duplication, bilateral asymmetry and tortuousity. Vernier calliper was used to note the size of the vessels and asymmetry of cerebral vessels of CW were assessed. It was not possible to measure the diameter of few specimens as size of fetal vessels was too small. In order to study the inter observer variability in measurements, we performed a paired "t" test between first and second measurement of the cases. Hypoplastic vessels were defined to be those with an external diameter of less than half the average. The observations were recorded as per the Performa. The results were tabulated and computed.
Results
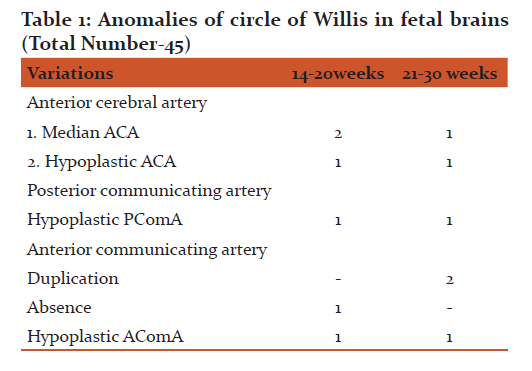
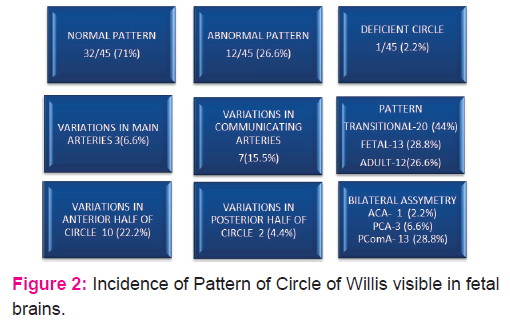
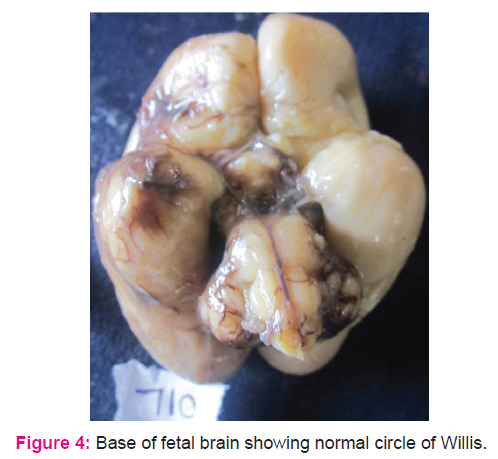
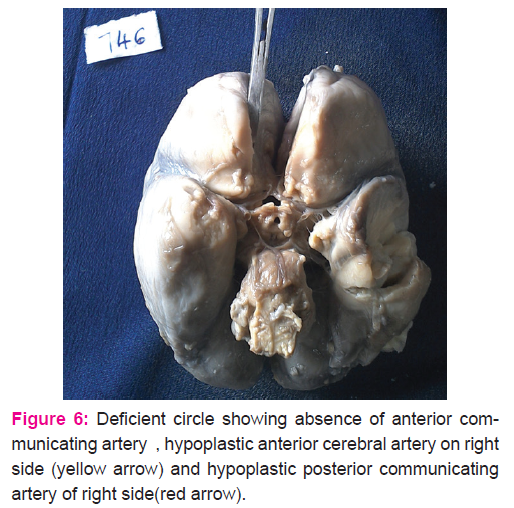
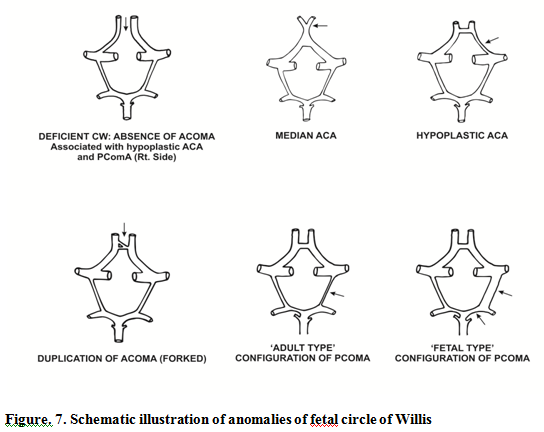
Forty five fetal brains aged between 14 and 30 weeks were dissected (Fig.1.) In 71% of fetal brains, the configuration was normal as described in textbooks (Fig .4.) Variations in CW were noted in 12 (26.6%) cases which included duplication of anterior communicating artery (Fig.5.), hypoplasia (of anterior cerebral artery, posterior communicating artery, anterior communicating artery) in 4.4% cases respectively, and a single median anterior cerebral artery as shown in Table.1. Absence of A ComA was also observed (Fig.6.) In 2 specimens (4.4%), the two ACAs join to form a single, median ACA (azygous ACA) which later on splits into two ACA. (Fig.7). In all these cases, the continuity of the anastomosis is maintained. However in one specimen the AComA was absent, therefore the circle was termed as deficient (Fig.2, 7).
The variations affect the anterior half of the circle (22.2%) more than posterior half (4.4%). Also the communicating vessels showed more variations as compared to other main cerebral arteries (Fig.2.). The most commonly duplicated or fenestrated artery was AcomA (Fig5.), present in 4.4% of the cases in the present study (Table.1). The variations appeared more frequently on the left side than the right side.
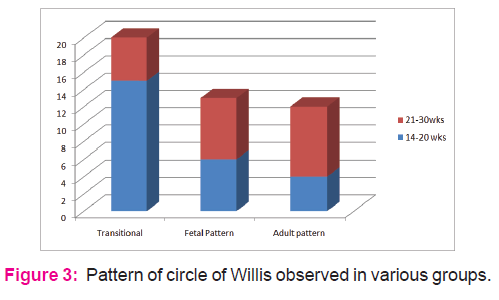
Fetuses from age groups of 14-20+ wks mostly showed presence of all component vessels and less asymmetry. The diameter of P com A and PCA were apparently same and the so called "fetal or embryonic pattern" was not visible. Fetuses from age groups of 21-30 wks mostly showed presence of all component vessels and more asymmetry. The diameter of either PcomA or PCA was larger. It looked like the standard "fetal or adult pattern" (Fig.7). The transitional configuration was more prevalent (66.6%) in 14-20+ wks ; in later age groups the transitional configuration was observed in only 33.3% and in other specimens changed to either adult (40%) or fetal ( 35%) type.
Discussion
The CW starts developing around 4th week (24th day) of IUL and by 7th week (CRL 40mm), fully formed, closed CW can be visualised3.The standard textbook of Embryology 4 describe that initially the Internal carotid artery (ICA) divides into Anterior, Middle, and Posterior cerebral arteries. The latter extends backwards and divides into two branches- the medial branch joins the Basilar artery while the lateral extends to supply the occipital and temporal lobes. The growth of occipital lobes and brain stem provides the initial directive for the development of vertebro-basilar system. In due course of time blood from the basilar artery starts circulating in the medial branch and from there in the lateral branch supplying the temporal and occipital lobes. On account of hemodynamic factors, the proximal part of the embryonic PCA up to its division becomes smaller in calibre and is now called the posterior communicating artery (PComA).The medial and lateral branches of the original PCA are now named as proximal and distal segments of PCA arising from the basilar artery. The anterior communicating artery (AComA) appears in the human embryo at (CRL 18 mm) as a reticulated anastomosis between the two ACAs. At 24mm CR length, this network fuses to form a single AComA3.
Functional demands of the growing brain tissue govern the development of cerebral vasculature. Soon with the regression of some earlier vessels and appearance of new channels, a fully developed CW becomes functional at the end of 6-7weeks. As the cerebral vascular tree reaches its typical configuration, the multiple events that occur during the embryological stage might lead to a diverse spectrum of vascular anatomical variants1. Duplications and fenestrations of an artery can occur due to an incomplete fusion of arteries 2. This has been associated with the development of cerebral saccular aneurysm5.The theory behind the fact is that smooth muscle cells and collagen are decreased at the proximal and distal end of the fenestrated segment. Even in patients of subarachnoid haemorrhage, 95% of the cases are found to have fenestrations close to the site of hematoma6.
De Silva et al, (2010)7 concluded that there is a significant shift in the contribution of carotid versus basilar system. In older fetal brains, blood flow from internal carotid artery is more in PCA which changes to gradually more contribution from vertebra-basilar in the adult brains. Ardakami et al, (2008) 8 dissected 30 fetal and infantile brains observed hypoplasia of both communicating vessels similar to the present study. Absence of PComA was also reported by them, however in the present study absence of AComA was observed. They concluded that CW configuration in their samples was similar to adults as most of the cases were from infants not from fetuses.
Overbeek and Hilton,(1991) 9 in their exhaustive study on fetal brains observed three types of configuration in relation to the contribution of vertebro-basilar trunk. According to them, in very early gestational period (12-21wks), the most prevalent pattern was transitional (73%) in which all cerebral vessels are slender but of equal size. After 20-21 wks, the transitional phase decreased and changed into either fetal or adult type of configuration, similar to the present study (Fig.3.). According to literature2, there is a fast growth of the occipital lobes around 22-24th weeks of IU life. It demands an increase in blood supply. So to satisfy the increased functional demands, either the PComA ( branch of ICA) or the PCA (branch of basilar artery) enlarge and from that time an adult or fetal configuration may develop. Because of hemodynamic factors, the most favoured configuration is more enlargement of PCA i.e. adult configuration. Therefore the configuration of newly formed CW looks symmetrical and slender; this can be assumed as transitional phase; In later half of IU Life, this transitional phase will directly change into either fetal or in adult type of configuration.
According to Menshawi, (2015)2 the primary goal of the developing cerebral arterial system in the embryo is to supply blood to support the rapid and asymmetrical brain growth. As long as the flow is delivered, there should not be a deleterious immediate consequence to the brain or the vessel. In the long term, however, individuals with arterial configurations consisting of decreased collaterals routes (e.g., lack of PComA or AComA) or coexistence of branching arteries with discrepant diameters may be at a higher risk of developing atherosclerosis in areas with chronic low or oscillating blood flow.
In view of above mentioned literature, the anatomical variants of the fetal CW were classified and compared in the present study. The variations affect the anterior half of the circle more than posterior half and variations appeared more frequently on the right side. In this aspect, the present study differs from Krishnamurthy et al. (2006)10 where posterior part of circle showed larger number of variations. The variations appeared more frequently on the left side in the present study. Also the communicating vessels showed more variations as compared to other cerebral arteries.
Absence of A ComA was also reported by Critchley (1930)11 and Milenkovic et al,(1985)1 similar to the present study. All other component cerebral vessels were present in our fetal specimens; which is not in accordance with some other studies 12. In 2 specimens (4.4%), the two ACAs join to form a single, median ACA (azygous ACA) which later on splits into two ACA. De Vriese, (1905)13 and Menshawi, (2015)2 has also confirmed the presence of single median ACA in fetus as observed in the present study. According to them this vessel is present normally in primates. Therefore its presence in human brains can be attributed to its repetition in phylogeny.
A vessel can be classified as hypoplastic if the diameter is less than half the average, although it was not possible to measure the diameter in fetal brains, the vessels were not patent while injecting a coloured solution through the vessel. Hypoplasia of anterior cerebral artery was detected in 4.4% of the cases comparable to series of Milenkovic et al, (1985)1 (9.6%) and Riggs (1983)14 (7-28%).
Duplicated or fenestrated arteries are the second most common variants (after hypoplastic arteries) and are reportedly more frequent in the anterior circulation15. The most commonly duplicated or fenestrated artery was AcomA , present in 4.4% of the cases in the present study in contrast to incidence of 32% observed by Milenkovic et al,(1985)1, and Ardkani et al,(2008)8. Fenestration or duplications might be due to an incomplete fusion of arteries during development, however some authors believe fenestrations can also be induced by the trans-arterial course of a nerve, a bony structure or enlarged vasa vasorum along the path of the artery2. The presence of such variations in the study (hypoplasia / duplication /fenestration/ absence) in fetal brains lead to hypothesis that these are congenital in origin.
Fetuses from age groups of 14-20+ wks mostly showed presence of all component vessels and less asymmetry. The diameter of P com A and PCA were apparently same and the so called "fetal or embryonic pattern" was not visible. Fetuses from age groups of 21-30 wks mostly showed presence of all component vessels and more asymmetry. The diameter of either PcomA or PCA was larger. It looked like the standard "fetal or adult pattern" in the present study. These observations are in agreement with Padget DH,(1948)3, Overbeek and Hilton,(1991)7, Ardkani et al,(2008)8 and Menshawi et al,(2015)2, i.e. transitional configuration was more prevalent (60%) in 15-20+ wks ; in later age groups the transitional configuration was observed in only 25% and in brains from other fetuses changed to either adult (40%) or fetal ( 35%) type. In a similar study Raamt et al, (2006)16 has defined a term ' 'FTP' (Fetal Type Posterior CW) as full or partial. They have stated that in full FTP the P1 segment of PCA (i.e. contribution from basilar artery) is absent, thereby making the collateral flow entirely dependent on the anterior circulation of opposite side. The present study did not find even a single case with complete absence of P1 segment of PCA. Patients with fetal variation of CW are prone to develop vascular insufficiency as there exists an embryological defect in primary collateral circulation between anterior and posterior half of the circle17.
The number and type of variations in fetuses were lesser as compared to adults compared to a previous study done by the same author18 .One of the striking features of fetal CW was the absence of tortuous vessels which is so evident in adult brains. The literature does not throw any light on this aspect. It is assumed that development of tortuousity might be the result of ever expanding brain which increased the demand for more blood supply. Subsequently the increase in length of vessels will make it tortuous as because of anastomosis, the vessel is tied at both ends.
Asymmetry in the two halves of circle was observed in only 37.8% fetal brains on account of unilateral hypoplastic vessels.
There was no incidence of saccular aneurysm in fetal CW in the present study. None has been reported in literature also; thereby indicating that occurrence of saccular aneurysm is not congenital.
Conclusion
Abnormal pattern was observed in 26.6% fetal brains; however the CW was deficient in only 2.2% specimens. Variations in the posterior part of CW are the result of developmental modifications. Absence / duplication of AComA is also the persistence of early fetal formation of this artery by a plexus. The presence of a single median (fused) ACA in fetus could not be explained on embryological basis. It might be due to the persistence of an arrangement normally found in lower primates. Tortuousity of cerebral vessels was not observed in fetal brains. Asymmetry on the two sides was also observed in only 37.8% fetuses.
There was no evidence of presence of aneurysms in fetal brains. The cause of development of aneurysms later in life could be (i) congenital defect in the arterial wall (ii) hemodynamic stress (iii) combination of both.
It is concluded that variations existing early in fetal life could be a consequence of genetic factors whereas variations developing during adult life could be due to mechanical stress factors. Their recognition is important as some of these might be responsible for the development of cerebral atherosclerosis, stroke or even saccular aneurysm.
Conflict of interest
The authors declare that they have no conflict of interest concerning this article.
No funding was required for data collection, data analysis and data interpretation of the study.
Acknowledgement
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are grateful to authors/editors/publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.


References:
1. Milenkovic Z, Vucetic R, Puzic M. Assymmetry and Anomalies of the Circle of Willis in fetal brain ,microsurgical study and functional remarks. Surg Neurol 1985;24:563-70.
2. Menshawi K, Mohr JP,and Gutierrez J. J Stroke 2015;17(2):144-58.
3. Padget DH. The circle of Willis: its embryology and anatomy. In: Dandy WE, Editor. Intracranial Arterial Aneurysms. New York. Comstock Publishing Company.1944.p:67-90
4. Hamilton WJ, Mossman HW. Cardiovascular system In : Hamilton, Boyd and Mossman's Human Embryology. Cambridge. The Williams and Wilkins Company 4th ed ;1972.p266-68.
5. LaBorde DV, Mason AM, Riley J, Dion JE, Barrow DL. Aneurysm of a duplicate middle cerebral artery. World Neurosurg. 2012;77:201.e1-201.e4.
6. Hudák I, Lenzsér G, Lunenkova V, Dóczi T. Cerebral arterial fenestrations: a common phenomenon in unexplained subarachnoid haemorrhage. Acta Neurochir (Wien). 2013;155:217-22.
7. De Silva KRD, Silva R, De Silva C, Gunasekera WSL, Dias P and Jayesekera RW. Comparison of the configuration of the posterior bifurcation of the posterior communicating artery between fetal and adult brains: A study of a Sri Lankan population. Ann Indian Acad Neurol.2010; 13(3):198-201.
8. Ardakani SK, Dadmehr M, Nejat F , Ansari S, Eftekhar B, and Tajik P et al. The cerebral arterial circle (Circulus Arteriosus Cerebri) A Anatomical study in fetus and infant samples.Pediatr Neurosurg. 2008;44:388-92.
9. Overbeeke JJV, Hillen B, Tulleken CAF. A comparative study of the circle of Willis in fetal and adult life. The configuration of the posterior bifurcation of the posterior communicating artery. J Anat.1991;176:45-54.
10. Krishnamurthy A,Rao CP, Naryana K, Nayak SR, Kumar SM, Surendran S. Circulus arteriosus cerebri: A study of variations in the fetal and adult human brains of south Indians. Morphologie. 2006; 90(290): 139-43
11 Critchley M. The anterior cerebral artery, and its syndromes. Brain. 1930;53:120-65.
12. Lavielle L,Choux M,Sedan R. Etude anatomo-radiologique de l'artere communicante posterieure. Neurochirugie. 1966; 12 :717-32.
13. De Vriese B.Sur la sygnification morphologique des arteres cerebrales. Arch Biol. 1905;21:357-457.
14. Riggs HE, Rupp C. Variations in form of circle of Willis. Arch Neurol 1963;8:8-14.
15. Van Rooij SB, Van Rooij WJ, Sluzewski M, Sprengers ME. Fenestrations of intracranial arteries detected with 3D rotational angiography. AJNR Am J Neuroradiol. 2009;30:1347-50.
16. Van Raamt AF, Mali VVP, Van Laar PJ and Van der Graaf Y. The fetal variant of the circle of Willis and its influence on the cerebral collateral circulation. Cerebrovac Dis. 2006;22(4):217-24.
17. Wu HM, Chuang YM. Acta Neurol Taiwan.2011;20(4): 232-42.
18. Kapoor K,Singh B and Late Jit Dewan I. Variations in the configuration of the circle of Willis. Anatomical Science International 2008;83:96-106.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License