IJCRR - 4(17), September, 2012
Pages: 47-51
Date of Publication: 14-Sep-2012
Print Article
Download XML Download PDF
STUDY OF LIPID PEROXIDATION, ANTIOXIDANT STATUS AND LIPID PROFILE IN BREAST CANCER
Author: G.S.R.Kedari, G.S.R.Hareesh, A. Saseekala
Category: Healthcare
Abstract:OBJECTIVE: Breast cancer is the commonest malignancy in women in India and western countries. Increased production of oxygen free radicals exhaust the antioxidant levels in the body leading to the development of oxidative stress which results in the production of cancer. The reasons for alteration of lipid profile in breast cancer is not clearly understood. The aim of our present study is to evaluate the role of oxidative stress and also the status of serum lipid profile in breast cancer patients. METHODS: The role of oxidative stress in breast cancer patients were evaluated by estimating the levels of lipid peroxidation assessing plasma Malondialdehyde(MDA) levels and antioxidant status by reduced glutathione(GSH), Vitamin-C in blood. We also tried to assess lipid profile by estimating Serum total cholesterol, triglycerides, HDL cholesterol and LDL cholesterol. For this, we have taken 50 cases of breast cancer patients compared with 50 age matched control women. RESULTS: There were significant increase in the levels of MDA and significant decreases in the levels of antioxidants like GSH and Vitamin-C in breast cancer patients when compared with controls. There were also significant increase in the levels of Serum total cholesterol, triglycerides and LDL cholesterol and no statistical significant difference was observed with HDL cholesterol in cases when compared with controls. CONCLUSION: Our results indicate that oxidative stress and alterations in lipid profile are associated with the development of breast cancer and the need for modification of lifestyle for the reduction of breast cancer development.
Keywords: Oxidative stress, Malondialdehyde, Reduced glutathione (GSH) and Vitamin-C.
Full Text:
INTRODUCTION
Breast cancer is one of the most common neoplasm?s in women and is a leading cause of cancer related deaths worldwide. The aetiology of breast cancer is multifactorial. Epidemiologic studies have identified many risk factors that increase the chance of a woman developing breast cancer include early age at menarche, late age of menopause, null parity, obesity, oral contraception, hormone replacement therapy, diet, family history, prior history of benign breast disease and lactation. The common denominator for many of these risk factors is their effect on the level and duration of exposure to endogenous or exogenous estrogens.
Oxidative stress is implicated in the pathogenesis of a variety of human diseases(1). Oxidative damage occurs to biomolecules like lipids, proteins, carbohydrates and nucleic acids and other extracellular components like collagen and hyaluronic acid which are very deleterious(2 )resulting in lipid peroxidation, mutagenesis and carcinogenesis. However, the body?s defense mechanisms play an important role in the form of antioxidants that help to minimize the damages which are caused by oxidative stress. Antioxidants are compounds that dispose, scavenge and suppress the formation of free radicals or oppose their actions. Oxidative stress occurs when there is an imbalance between reactive oxygen species(ROS) and antioxidants reaction capacity which stimulate the development of a disease such as breast cancer(3).Several case control studies have reported a relationship with antioxidant status(4,5) and a reduction in antioxidant level due to the presence of free radicals may increase risk of breast cancer. The neoplastic disease is related to new growth where there is greater utilization of lipids including total cholesterol, lipoproteins, and triglycerides for new membrane biogenesis. Cells fulfill these requirements either from circulation, by synthesis through the metabolism or from degradation of major lipoprotein fractions like VLDL, LDL or HDL(6). The aim of our study was to evaluate the role of lipid peroxidation in breast cancer by estimating the plasma MDA levels and the role of enzymatic and non enzymatic antioxidants by estimating reduced glutathione and vitamin-C and also to evaluate the relationship between the lipid profile and breast cancer.
MATERIAL AND METHODS
The present study was conducted in the department of biochemistry and department of general surgery in Saveetha medical college and S.V. Medical college, Tirupati.50 newly diagnosed cases of breast cancer belonging to age group of 30-70 years were included in this study. Out of cases,43 were having ductal carcinoma and 6 patients were having lobular carcinoma and 1 was having mixed carcinoma(both ductal and lobular).75 age matched women who have no history of breast diseases were taken as controls. All the subjects were not using any kind of hormonal therapy and they had no any other diseases like Diabetes mellitus, liver diseases and thyroid disorders. Informed consent was obtained from all the subjects. Due permission was obtained from Ethical clearance committee for this study. 10ml of fasting blood samples were collected by venipuncture and for the separation of sera, 5ml of blood was centrifuged at 3000rpm for 5min and the remaining 5ml of blood was taken into a plain vial containing EDTA and was centrifuged at 3000rpm for 10min for the separation of plasma. The plasma MDA levels were estimated by using thiobarbituric acid reacting substances(TBARS) by the method of Yagi(7) and Sinnhuber et al(8).Reduced glutathione was determined by the method of Beutler et al(9). The activity of Ascorbic acid was determined by the method of Tietz(10).Serum was used for the estimation of lipid profile. Total cholesterol and triglycerides were estimated by enzymatic methods(11,12).HDL cholesterol(HDL-C) was estimated by phosphotungstic acid precipitation followed by enzymatic analysis in supernatant fraction(13) and LDL-cholesterol was determined by using Friedwald?s equation(14). All the results were expressed as mean ± SD and statistical comparisons were done using student t-test using the SPSS package.

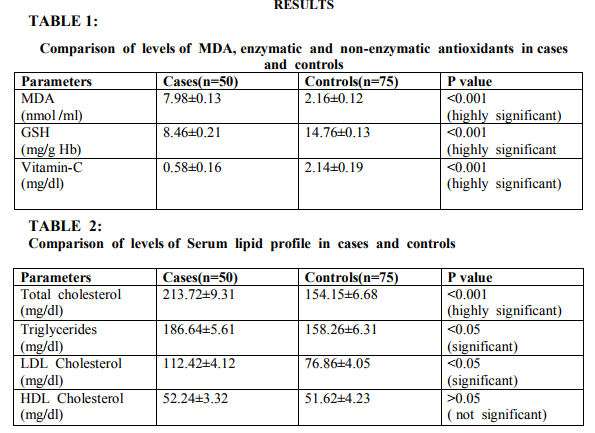
Evaluation of oxidative stress is done based on the levels of MDA and statistically significant increase in the level of MDA was observed in breast cancer patients when compared to controls. Statistically significant decreases were observed in the levels of antioxidants like GSH and Vitamin-C in cases when compared to controls. There were also significant increases in the levels of serum total cholesterol, triglycerides and LDL cholesterol in cases when compared to controls. There was no statistical significant difference in the levels of HDL in cases when compared with controls.
DISCUSSION
Damage to the breast epithelium by oxygen free radicals can lead to fibroblast proliferation, epithelial hyperplasia, cellular atypia and breast cancer. Studies have shown increased lipid peroxidation in solid tumors(15,16).The significant rise in the MDA levels in breast cancer confirms that it is associated with an increased production of reactive oxygen species(ROS) and free radicals. Lipid peroxidation is a chain reaction which provides a continuous supply of free radicals that initiate further peroxidation(17). We also observed a significant decrease in the levels of reduced glutathione in the cases as compared to the controls. The intracellular depletion of reduced glutathione can be either due to the formation of a direct complex with a electrophilic agent or due to the inhibition of synthesis or due to the subjection of the cell to oxidative stress(18). When a cell is subjected to oxidative stress, there is increased utilization of glutathione, thus leading to its depletion. Many enzymes are GSH dependent and their activity may be regulated by the thiol disulphide exchange. They are thus dependent on the GSH status. Glutathione –S- transferase (GST) is reduced in diabetics which are dimeric, mainly cytosolic enzymes that have extensive ligand binding properties in addition to their catalytic role in detoxification(19,20). This reduction is due to the reduced levels of GSH. There is also a decrease in the levels of non-enzymatic anti-oxidants such as Vit-C, which states that there is an increased defense mechanism against oxidative damage in breast cancer. The decrease in the levels of these non-enzymatic antioxidant parameters may be due to an increased turnover for preventing oxidative damage in these patients, thus suggesting an increased defense against oxidative damage. Our results support the researchers who reported decreases in the antioxidant level and increases in lipid peroxidation level(21,22).Several other researchers showed over expression of antioxidants(16,23). In the present study, there is significant increase in the levels of serum total cholesterol, LDL cholesterol and triglycerides in the breast cancer patients when compared with normal subjects and there is no significant difference in the levels of HDL cholesterol . The patho physiological mechanism for lipid alterations underlying is not well understood. Lipids are major cell membrane components essential for various biological functions including cell growth and division of normal and malignant tissues. Low levels of cholesterol in the proliferating tissues and in blood compartments could be due to the process of carcinogenesis. The raised plasma concentrations of theses parameters in patients with breast cancer may be due to an increased rate of lipid absorption as the fat splitting enzymes, lipases, were also found to be increased in the patients(24). In conclusion, the present study demonstrated high oxidative stress, low antioxidant status with rise in plasma lipid levels except HDL in breast cancer patients which shows the need to adopt a healthy lifestyle to reduce oxidative stress with consumption of diet rich in antioxidant nutrients to prevent breast cancer.
CONCLUSION
Our results indicate that oxidative stress and alterations in lipid profile are associated with the development of breast cancer and the need for modification of lifestyle for the reduction of breast cancer development.
ACKNOWLEDGEMENTS
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors/editors/publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
1.Beck MM, Levander OA. Dietary oxidative stress and potentiation of viral infection. Annu Rev Nutr,1998; 18: 93-116.
2.Frei B. Reactive oxygen species and antioxidant vitamins: mechanisms of action. Am J Med,1994; 97: S5-S13.
3.Aghvami T, Djalali M, Kesharvarz A, et al. Plasma level of antioxidant vitamins and lipid peroxidation in breast cancer patients. Iran J Publ Health,2006; 35: 42-7.
4.Ching S, Ingra D, Hahnel R, Beilby J, Rossie E. Serum levels of micronutrients, antioxidants and total antioxidant status predict risk of breast cancer in a case control study. J Nutr, 2002; 132: 303-6.
5.Do MH, Lee SS, Jung PJ, Lee MH. Intake of dietary fat and vitamin in relation to breast cancer risk in Korean women: a case control study. J Korean Med Sci,2003; 18: 534-40.
6.M.I.Qadir, S.A.Malik. Plasma lipid profile in gynecologic cancers. Eur.J.Gynaec.oncol, 2008; 29: 158-161.
7.Yagi K. Lipid peroxides and human diseases. Chem Phys Lipids, 1978; 45: 337-351.
8.Sinnhuber RO, Yu TC. Characterization of red pigment formed in thiobarbituric acid determination of oxidative rancidity. Food Res,1958; 23: 626-630.
9.Beutler E, Duron O, Kelly BM. Improved method for determination of blood glutathione. J Lab Clin Med, 1963;61:882-888.
10. Tietz NW(Ed). Textbook of Clinical Chemistry. W.B.Saunders Company, Philadelphia, London, Toronto.2004; pp.960-962.
11. Alian CC,Poon LS, Chan CSG. Enzymatic determination of total serum cholesterol. Clinical Chemistry.1974; 20; 470-475.
12. Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clinical chemistry. 1973;19:476-482.
13. Burstein M, Scholnick HR, Morfin R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyamines. Journal of lipid research,1970;2:583-595.
14. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma without the use of preparatory centrifuge. Clin Chem 1972; 18: 499-503.
15. Zieba M, Nowak D, Suwalski M,et al. Enhanced lipid peroxidation in cancer tissue homogenates in non small cell lung cancer.Monaldi Arch Chest Dis 2001;56:110-4.
16. Skrzydlewska E, Stankiewicz A, Sulkowska M, Sulkowski S, Kasacka I. Antioxidant status and lipid peroxidation in colorectal cancer. J Toxicol Environ Health 2001;64:213- 22.
17. Murray RK, Granner DK, Rodwell VW. Harper?s Illustrated Biochemistry. 27th Edn.2006.Mc Graw Hill Lange International Edition.pp128-129.
18. Deneke SM, Farberg TZ. Regulation of cellular glutathione lung cells. Mol.Physiol,1994 ;1163-1173.
19. Listowsky I,Abramovitz M, Homma H et al. Intracellular binding and transport of hormones and Xenobiotics by glutathione-Stransferase. Drug Metab Res,1988;19:305-318.
20. 20.Ketley JN, Habig WH, Jacoby WB. Binding of non substrate ligands to glutathione-s-transferases. J Biol Chem,1976;250:8670-8673.
21. Gonenc A, Erten D, Aslan S, et al. Lipid peroxidation andantioxidant status in blood and tissue of malignant breast tumor and benign breast disease. Cell Biol Int 2006;30:376-80.
22. Yeh CC, Hou MF, Tsai SM, et al. Superoxide anion radical, lipid peroxides and antioxidant status in the blood of patients with breast cancer. Clin Chim Acta 2005;361:104- 11.
23. Iscan M, Coban T, Cok I, et al. The organochlorine pesticide residues and antioxidant enzyme activities in human breast tumors: is there any association? Breast Cancer Res Treat, 2002;72:173.
24. Basu T.K and Williams D.C. Plasma and body lipids in patients with carcinoma of the breast.Oncol.,1975;31:172.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License