IJCRR - 9(16), August, 2017
Pages: 49-57
Print Article
Download XML Download PDF
Alterations in Carotid Body Morphology and Cellular Mechanism Under the Influence of Intermittent Hypoxia
Author: Utkarsha Kumar, Dishari Ghosh, Snigdha Shaw, Gopinath Bhaumik, Rajinder K Gupta, Prasanna K Reddy, Shashi Bala Singh
Category: Healthcare
Abstract:Aims: Intermittent hypoxia (IH) training is said to have a preconditioning effect for evoking acclimatization at high altitude (HA). Carotid body (CB) plays a vital role in oxygen sensing and is an important component in HA acclimatization. The present study reports the mechanistic effects of IH that involves episodes of hypoxia of few hours continued for several days, on the CB responses to acute hypobaric hypoxia in terms of morphological changes in CB and its cellular functions.
Methodology: 24 Sprague-Dawley (250-300g) rats were divided into 2 major groups: 1) control, 2) experimental group (n=12 each) in which the rats were exposed to IH training for 10 days with a single hypoxic episode of 4h/day at a simulated altitude of 15000ft. 6 rats from each group were further subjected to a simulated hypobaric acute hypoxic (AH) challenge of 1hr at 25000ft to see the effect of IH training (IHT) and were named as 3) Control+ AH challenge and 4) IHT+ AH challenge. Morphological changes in CB in different groups were observed along with expression of hypoxia inducible factor (HIF) 1\a, HIF2\a, NADPH Oxidase 2 (NOX2) and Superoxide Dismutase 2 (SOD2) using immunohistochemistry for the first time.
Results: The results showed that IH training leads to morphological changes in terms of hyperplasia and unaltered HIF1\a levels along with a highly significant rise in HIF2\a in CB. When the rats are exposed to AH without IH conditioning, there is a significant rise in HIF1\a and thus NOX2 levels. However, prior exposure to IH leads to a significant rise in the HIF2\a levels and thus SOD2 levels, when subjected to AH challenge.
Discussion and Conclusion: These results indicate that IH training affects the cellular response of CB by regulating balanced expression of both HIF1\a and HIF2\a, thus modulating the cellular redox state by promoting the antioxidant enzyme production and suppressing the pro-oxidant enzyme levels, thereby playing a crucial role in pre-conditioning to acute hypoxia.
Keywords: Carotid Body, Intermittent hypoxia, HIF1?, HIF2?, NOX2, SOD2
DOI: 10.7324/IJCRR.2017.9169
Full Text:
Introduction
Carotid bodies (CBs) are small neurovascular structures at the bifurcations of the common carotid arteries comprising of Type I (chief or glomus) and type II (sustentacular) cells [1] that recognizes the oxygen (O2) deprivation (hypoxia) in the arterial blood. Various morphological changes have been reported in mammalian CB on chronic as well as on acute hypoxic exposure. CBs protect the organs from hypoxic damage by releasing neurotransmitters (NTs) and instantaneously signaling the brainstem respiratory centre via the carotid body nerve, resulting in hyperventilation that is an integral part of altitude acclimatization [2-4]. Currently, models of oxygen sensing are based on either a heme protein or the production of reactive oxygen species (ROS) by NAD(P)H oxidases and mitochondria [5]. Other than these, HIF1 and HIF2, members of hypoxia inducible factor (HIF) family are believed to be chief molecular determinants for CB O2 sensing. They constitute a common HIFβ subunit with oxygen regulated HIF1α and HIF2α subunits respectively [6,7]. HIF1α is a potent activator of genes encoding pro-oxidant enzymes such as NADPH oxidase 2 (NOX2) [8] and HIF2α regulates antioxidant enzymes such as superoxide dismutase 2 (SOD2) [9]. The cellular redox state relies on the regulation of these enzymes by the respective HIFα isoforms that further triggers the signaling pathways such as the release of multiple NTs [10,11].
Hypoxia and intermittent hypoxia (or IH i.e. repeated episodes of hypoxia interspersed with normoxic or less severe hypoxic episodes) mediate oxygen sensing in the CBs. It has been well documented that IH as in obstructive sleep apnea (OSA) cases (hypoxia for 10-40 seconds, normoxia of various minutes) [10] showed no significant effect on the morphology of CB, be it CB hypertrophy or hyperplasia of glomus cell volume, though it increases CB’s sensitivity and toxic sympathetic activation [12]. Moreover, studies reveal that augmented activity of the CB, induced by IH plays an important role in the pathogenesis of sleep apnea [13] in anesthetized rats and humans and emphasis is being made to understand its underlying mechanisms. Furthermore, the equilibrium between HIF1α and HIF2α, hampered by chronic IH leads to an imbalance between pro-oxidant and antioxidant enzymes respectively that causes oxidative stress resulting in pathophysiology [14-16] and has been implicated as a risk factor for an array of cardiovascular diseases [17] affecting humans.
IH training (IHT) is being extensively used by sports medicine community to improve performance and by the US army to induce pre-acclimatization without any pharmacological interventions [18]. A low number of re-oxygenation cycles per day and moderate hypoxic episodes elicits the beneficial effects beyond pathology and provides protection against hypertension, myocardial injuries, heart arrhythmia and bronchial asthma [19,20]. It helps in activating the surviving mechanisms against acute hypoxic exposure by increasing haemoglobin, erythropoietin production, total red blood cells, exercise time, hypoxic ventilatory responses in healthy human subjects, bone mineral density, respiratory and non respiratory somatic motor recovery following spinal injuries in rats and humans and enhancement of spatial learning and memory without any detectable adverse consequences [21].
The effect of the paradigm of IH resembling recurrent apneas and the role of CB in the disease mechanisms has been studied considerably in the recent years. Also, studies have been focused upon the IHT consisting of hypoxic episodes of few hours in human subjects. However despite these aforesaid extensive studies, the pre-conditioning effect of IH of long duration on the rat CB and the probable cellular mechanism whereby IH mediates the CB activity remains unstudied. In the present study, we evaluate the mechanistic effects of IH on CB responses to acute hypoxia in rats, in terms of changes in CB morphology and cellular functions.
Materials and Methods
Animals
Male Sprague-Dawley rats (250-300g) were used for all experiments that were maintained under a 12-h light–dark cycle at temperature 25±1°C, humidity 55% ± 2%, in the Institute’s animal house facility. The study was approved by the Animal Ethical Committee of the institute in accordance with Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA) of the Government of India. All efforts were made to minimize the animal suffering and to reduce the number of the animals used.
Experimental Design and Hypobaric Hypoxia Exposure
Animals were randomly assigned to two main groups:
- Control (n=12) rats maintained in normoxic condition.
- Experimental group (n=12) rats subjected to intermittent hypobaric hypoxia (IH) with hypoxic episode of 4h/day at 15000ft for 10 days [13] (with modifications) in animal decompression chamber. The airflow in the chamber was 2L/min with relative humidity 40%-50% and temperature 25±1?C.
6 rats from each of these groups were exposed to an acute hypoxic challenge (AH) at simulated altitude of 25000ft for 1h to assess the effect of IH conditioning on acute hypobaric hypoxic exposure and to see whether the type of IH modality including longer durations of hypoxic exposure has some beneficial role against the deleterious effects of hypoxia or not. The groups were named as Control+ Challenge (C+AH) and IH+ Challenge (IHT+AH) respectively.
General preparations of the animals and tissue
After the hypoxic exposure, rats were anaesthetized with ketamine/xylazine (70 and 6mg/kg, i.p., respectively). An anterior neck incision was made and various neck muscles were retracted to expose carotid bifurcation along with CB. The tissue was perfused transcardially first with 0.05M phosphate buffer saline (PBS), followed by 4% paraformaldehyde in O.1M phosphate buffer, pH 7.3. After perfusion, the CBs were immediately excised and were post-fixed in the same fixative overnight at 4?C.
Histological Studies
The fixed tissues were dehydrated, cleared and embedded in paraffin blocks and 5-6 µm thick tissue sections were obtained using a rotary microtome that were stained with haematoxylin and eosin. The sections were examined under the light microscope.
Immunohistochemical Studies
The sections were dewaxed with xylene and rehydrated with a series of decreasing grade of ethanol solutions. They were then immersed in antigen retrieval solution (0.1M citric acid buffer, pH 6.0) for 10 min in water-bath (100?C) and to block endogenous peroxidase activity, they were immersed in 4% hydrogen peroxide for 5 min at room temperature (RT). Sections were pre-incubated (2h) with blocking serum to reduce non-specific binding of the antiserum. Between the separate steps, the sections were rinsed with cold PBS and incubated with the following primary antibodies: HIF1α (mouse monoclonal IgG antibody, 1:500 dilution, Sigma Life Science); SOD2 (rabbit polyclonal IgG antibody, 1:1500 dilution, Sigma Life Science); HIF2α (mouse monoclonal IgG antibody, 1:200 dilution, GeneTex) and NOX2 (rabbit polyclonal antibody, 1:200 dilution, PromoKine), in 0.05M Tris/HCl buffer, overnight at 4?C. They were washed three times in PBS and incubated (30 min) at RT with respective secondary antibody labeled with horseradish-peroxidase. Finally, sections were washed and the peroxidase was visualized by immersing in 0.05% diaminobenzidine (DAB) for 3-5 min, rinsed in distilled water and counterstained mildly with haematoxylin, dehydrated in graded alcohols, cleared in xylene, mounted with DPX solution and air-dried. Positive staining was indicated by a brown color.
Image acquisition
Histological and immunohistochemical images were acquired using a Leica DMR (Germany) microscope via an inbuilt CCD Color Camera (Leica) with 40X objective lens.
Image analysis
The section with the largest area of cells was chosen to perform measurements to evaluate the following parameters:
- Morphometric analysis was performed with NIH Image J software program.
- The total number of type I cells in the CB were counted using a mechanical cell counter in Image J with 40X objective lens.
- Values of integrated density (product of Area and Mean Gray Value of the pixels in the image) of immunohistochemical images of different groups were obtained using Image J (selected using the freehand selection tool) in order to quantify the expression levels of HIF1α, NOX2, HIF2α and SOD2 by averaging their respective values.
- Histogram profiles of the images were obtained by Image J by digital image analysis that is a plot of the intensity values of the pixels (x axis) vs. the number of pixels representing the intensities (y axis) [22]. The pixel intensity values for any color ranges from 0 to 255 (0 represents darkest color shade; 255 represents lightest color shade as standard). The profiling is done using the averaged values of the number of pixels in respective values of intensities of various cells in the images in a particular group. The increase or decrease in the intensities of DAB (brown color) is analyzed by observing the shift of the histogram of hypoxic exposed groups to the left or right of the histogram obtained in the control group.
Statistical analysis
Values were expressed as mean ± SD. Student’s t test was carried out for statistical analysis using SPSS for windows (15.0) software (SPSS Inc., Chicago, II). For all statistical evaluations, P values < 0.05 and <0.001 were considered statistically significant and extremely significant respectively.
Results
Morphology
Haematoxylin-eosin staining of the carotid body sections of control animals revealed a highly vascularized CB structure and loose irregularly arranged connective tissue with abundant capillaries. The chemoreceptor tissue possessed numerous, round shaped, specialized type I cells that contained a single prominent nucleus. It also had type II cells dispersed between type I cells, fusiform shaped whose extensions enveloped the glomus cells. They had an elongated nucleus and were deeply stained. It also comprised of several small nerve bundles that had the slightly wavy course of the axon (Fig 1).
The effects of the 10-day IHT were seen to be associated with the structural changes in the CB. The changes in the chemoreceptor tissue after giving IHT were in terms of hyperplasia, with an approximate 35% increase in the number of glomus cells when compared with the control group. The cell number of the CB tissue, however, remained unchanged in the control rats exposed to acute hypoxic challenge of 1h when compared with the control rats. No change in the size of the chief cells was observed in the CBs of different groups. Also, a decrease in the connective tissue was found in the CBs subjected to intermittent hypoxia of 10 days.
Immunohistochemistry
After comparing integrated densities of various antibodies, no difference of HIF1α levels was observed between the control and IH rats. However, the levels of HIF1α were significantly increased by 8% (approximately) in rats given AH only, when compared with control group. Also, the levels were 12% higher in these rats when compared with IHT+AH group (Fig 2A).
In case of HIF2α, there was a sharp increase of 15% in HIF2α levels in the IH group and a significant rise of 11% increase in the IHT +AH group when compared to control (Fig 3A).
The levels of pro-oxidants i.e. NOX2 was the highest in the group where no IHT was given to the rats before exposing them to AH. There was a significant rise in this group with an increase of 7% when compared to the control rats. There was no such elevation seen in the pro-oxidant levels in the rats that were preconditioned with IHT followed by AH challenge. Also no change in the NOX2 levels was observed in rats after giving IHT for 10 days when compared with control. Histogram profile of NOX2 in CBs showed that the intensity values of the pixels in the image started to appear much earlier in control and IH conditioned rats after giving AH. The histograms of these two groups were shifted to the left when compared to control. However, the histogram of IHT group was shifted to the right and appeared much later when compared with control. This implies that the DAB intensities were higher in AH-exposed rats and lesser in IH trained rats when compared to control (Fig 4 C).
In contrast, the antioxidant levels (SOD2) were highest in the rats given IHT. When compared with control group, there was a highly significant elevation of an approximate 12% in the SOD2 levels in these rats when compared to control. On giving 1h AH to these IHT preconditioned rats, the SOD2 levels were raised by 9% as compared to control. Upon exposure to AH without giving any IHT, there was no significant rise in the antioxidant enzyme levels observed (Fig 5A). In the case of SOD2 the histogram profiling showed that the DAB intensities were highest in rat CBs exposed to AH after IHT when compared to control (Fig5C). The DAB intensities were the lowest in control. The histogram of images obtained from IHT+AH group rats appeared first and was to the left of the control group histogram.
Discussion
The present study demonstrates that conditioning the rats with IH of few hours of hypoxic episodes leads to changes in the morphology of the CBs by hyperplasia of chief cells. CBs from IH trained rats showed increased levels of the two HIF isoforms when subjected to AH. However, HIF2α is strongly induced by the IHT that further regulates the expression of the antioxidant enzyme, SOD2. Further, no significant change was observed in the HIF1α levels of CBs pre-conditioned with IH in comparison to the control. To the best of our knowledge, from these findings we demonstrate for the first time that IHT used in this study affects the CB cellular response by regulating the expression of the HIFα isoforms and modulating the antioxidant enzyme levels that may further lead to alteration and maintenance of the cellular redox state. This maintenance of redox homeostasis in the CBs of IH trained rats enables them to sustain the AH stress.
Edwards et al [23] along with Prabhakar and Jacono [24] demonstrated the effect of chronic hypoxia on the CBs of various mammals that were born and lived in Peru (4330m) and showed the enlargement of their CBs when compared with sea level animals which was assumed to be due to hyperplasia of the glomus cells having vacuolated cytoplasm. The chronic hypoxia induced morphological changes in mammalian CB included enlargement of CB and chief cells, hyperplasia and mitosis of type I cells along with congestion of blood capillaries, marked vasodilation and neovascularisation. It has also been established that there is an increase in the number and diameter of glomus cells on chronic exposure to simulated HA and rapid enlargement of the rat CBs on AH exposure that appeared to be simply due to vascular congestion [3,4]. On the other hand, IH with shorter hypoxic bouts as seen in OSA model, though sustained for 10 days, showed an increase in the CB’s sensitivity and toxic sympathetic activation without obvious morphological alterations [12]. However, our results indicate 10 days of IH exposure to longer hypoxic episodes (4h/day) leads to hyperplasia of glomus cells by approximately 35% suggesting a CB O2 sensing mechanism different from OSA model. It is possible that the alterations in the glomus cell number might be contributing in part to elevate the NT release along with increased expression of transcriptional factors such as HIF1α and HIF2α in them to facilitate the O2 sensing process by CB at HA.
In the present study, using immunohistochemistry we have demonstrated the HIF1α and HIF2α expression in the rat CB. Previous workers have shown that HIF1α is found ubiquitously in vivo whereas HIF2α is tissue specific and is upregulated in specific cells of different organs
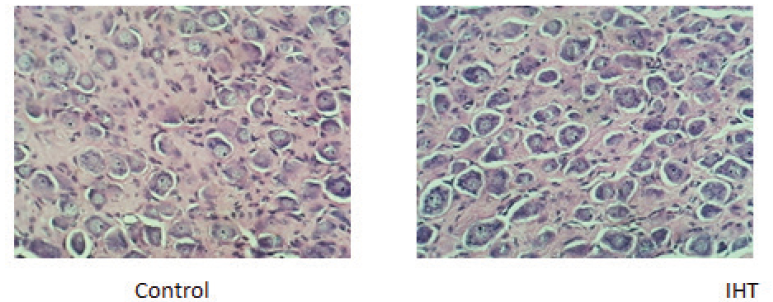
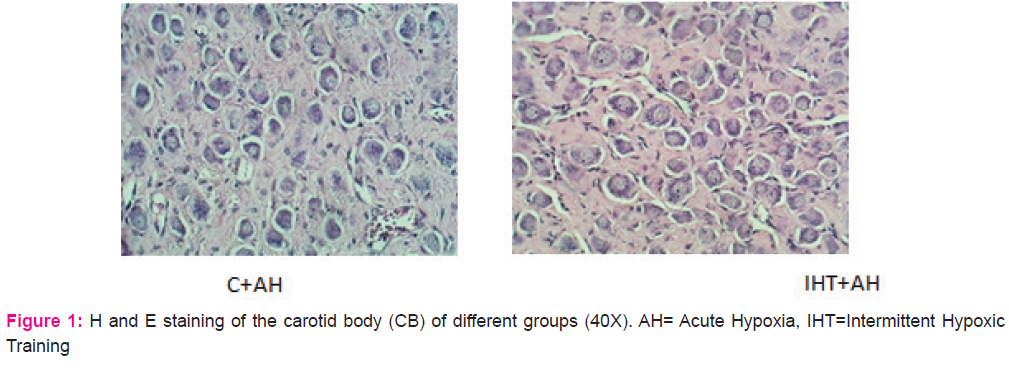

Figure 2. Integral density analysis of HIF1α using immunohistochemical images of CBs of various groups. A: Analysis of integrated density of HIF1α in rat CBs of various groups. Values are mean±SD (n=6). *P<0.05 compared with control group. B: Immunohistochemical expression of HIF1α in carotid bodies of rats exposed to hypobaric intermittent hypoxia at 15000ft for 4h/day for 10 days and acute hypoxia at 25000ft for 1h. The figure is representative of atleast 3-4 animals from each group. The signals were detected by DAB staining. Magnification: 40X. INT DENS= Integrated Density, C=Control, AH= Acute Hypoxia, IHT=Intermittent Hypoxic Training
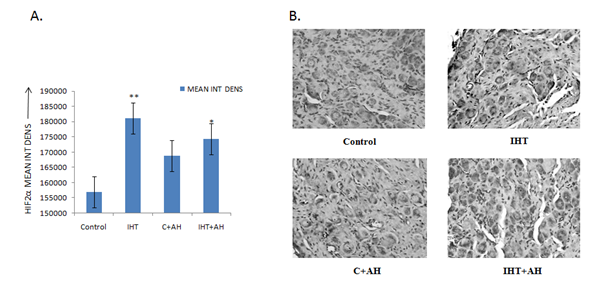

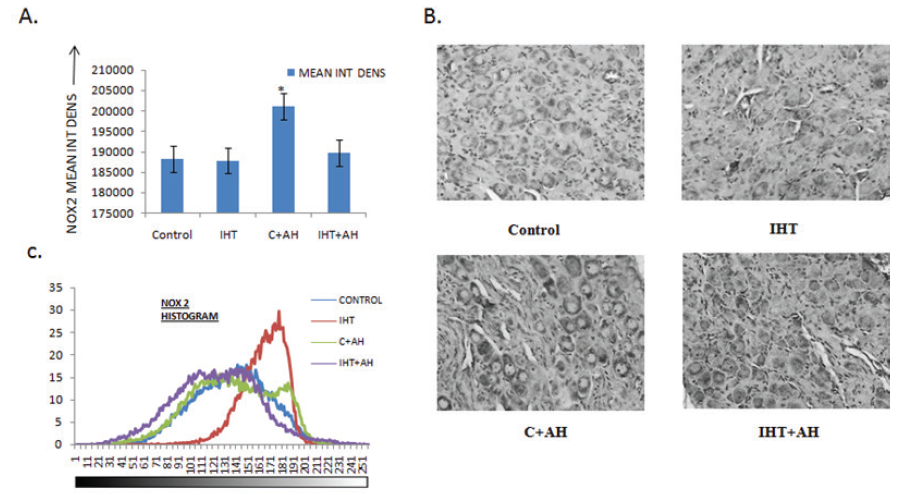
Figure 4: Integral density analysis and representative histogram profile of NOX2 in images of CBs of different groups by Image J. A: Analysis of integrated density of NOX2 in rat CBs of various groups. Values are mean±SD (n=6). *P<0.05 compared with control group. B: Immunohistochemical expression of NOX2 in carotid bodies of rats exposed to hypobaric intermittent hypoxia at 15000ft for 4h/day for 10 days and acute hypoxia at 25000ft for 1h. C: Representative histogram profile that corresponds to the pixel intensity value vs. corresponding number counts of pixel intensity. The figure is representative of atleast 3-4 animals from each group. The signals were detected by DAB staining. Magnification: 40X. INT DENS= Integrated Density , C= Control, AH= Acute Hypoxia, IHT=Intermittent Hypoxic Training.
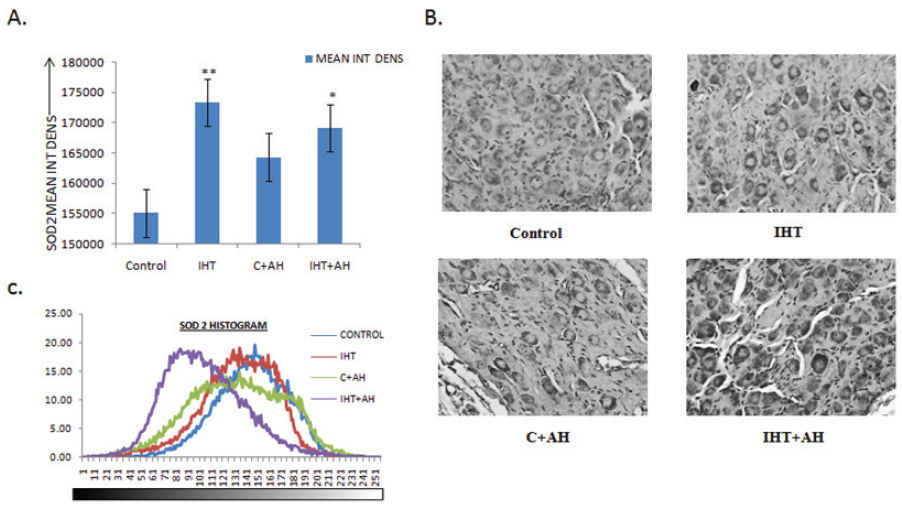
Figure 5. Integral density analysis and representative histogram profile of SOD2 in images of CBs of different groups by Image J. A: Analysis of integrated density of SOD2 in rat CBs of various groups. Values are mean±SD (n=6). *P<0.05, **P<0.001 compared with control group. B: Immunochemical staining for SOD2 distribution in rats exposed to acute hypoxia (1h) or control (without hypobaric hypoxia) with or without IH training. C: Representative histogram profile that corresponds to the pixel intensity value vs. corresponding number counts of pixel intensity. The figure is representative of atleast 3-4 animals from each group. The signals were detected by DAB staining. Magnification: 40X. INT DENS= Integrated Density, C=Control, AH= Acute Hypoxia, IHT=Intermittent Hypoxic Training
References:
1. Kay, J.M., Laidler, P.: Hypoxia and the carotid body. Journal of clinical pathology. Supplement (Royal College of Pathologists) 11, 30-44 (1977).
2. Dhillon, D.P., Barer, G.R., Walsh, M.: The enlarged carotid body of the chronically hypoxic and chronically hypoxic and hypercapnic rat: a morphometric analysis. Quarterly journal of
experimental physiology (Cambridge, England) 69(2), 301-317 (1984).
3. Lahiri, S., Rozanov, C., Cherniack, N.S.: Altered structure and function of the carotid body at high altitude and associated chemoreflexes. High altitude medicine and biology 1(1), 63-74
(2000). doi:10.1089/152702900320694
4. Wang, Z.Y., Bisgard, G.E.: Chronic hypoxia-induced morphological and neurochemical changes in the carotid body. Microscopy research and technique 59(3), 168-177 (2002).
doi:10.1002/jemt.10191
5. Lopez-Barneo, J., Pardal, R., Ortega-Saenz, P.: Cellular mechanism of oxygen sensing. Annual review of physiology 63, 259- 287 (2001). doi:10.1146/annurev.physiol.63.1.259
6. Semenza, G.L.: Hypoxia-inducible factors in physiology and medicine. Cell 148(3), 399-408 (2012). doi:10.1016/j. cell.2012.01.021
7. Yuan, G., Peng, Y.J., Reddy, V.D., Makarenko, V.V., Nanduri, J., Khan, S.A., Garcia, J.A., Kumar, G.K., Semenza, G.L., Prabhakar, N.R.: Mutual antagonism between hypoxia-inducible factors 1alpha and 2alpha regulates oxygen sensing and cardio-respiratory homeostasis. Proceedings of the National Academy of Sciences of the United States of America 110(19), E1788-1796 (2013). doi:10.1073/pnas.1305961110
8. Yuan, G., Khan, S.A., Luo, W., Nanduri, J., Semenza, G.L., Prabhakar, N.R.: Hypoxia-inducible factor 1 mediates increased expression of NADPH oxidase-2 in response to intermittent hypoxia. Journal of cellular physiology 226(11), 2925-2933 (2011). doi:10.1002/jcp.22640
9. Nanduri, J., Wang, N., Yuan, G., Khan, S.A., Souvannakitti, D., Peng, Y.J., Kumar, G.K., Garcia, J.A., Prabhakar, N.R.: Intermittent hypoxia degrades HIF-2alpha via calpains resulting
in oxidative stress: implications for recurrent apnea-induced morbidities. Proceedings of the National Academy of Sciences of the United States of America 106(4), 1199-1204 (2009).
doi:10.1073/pnas.0811018106
10. Prabhakar, N.R.: Oxygen sensing during intermittent hypoxia: cellular and molecular mechanisms. Journal of applied physiology (Bethesda, Md. : 1985) 90(5), 1986-1994 (2001).
11. Majmundar, A.J., Wong, W.J., Simon, M.C.: Hypoxia inducible factors and the response to hypoxic stress. Molecular cell 40(2), 294-309 (2010). doi:10.1016/j.molcel.2010.09.022
12. Peng, Y.-J., Overholt, J.L., Kline, D., Kumar, G.K., Prabhakar, N.R.: Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: Implications for recurrent apneas. Proceedings of the National Academy of Sciences of the United States of America 100(17), 10073-10078 (2003). doi:10.1073/ pnas.1734109100
13. Peng, Y.-J., Prabhakar, N.R.: Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. Journal of Applied Physiology 96(3), 1236-1242 (2004). doi:10.1152/ japplphysiol.00820.2003
14. Cave, A.C., Brewer, A.C., Narayanapanicker, A., Ray, R., Grieve, D.J., Walker, S., Shah, A.M.: NADPH oxidases in cardiovascular health and disease. Antioxidants and redox signaling
8(5-6), 691-728 (2006). doi:10.1089/ars.2006.8.691
15. Pendyala, S., Natarajan, V.: Redox regulation of Nox proteins. Respiratory physiology and neurobiology 174(3), 265-271 (2010). doi:10.1016/j.resp.2010.09.016
16. Rahal, A., Kumar, A., Singh, V., Yadav, B., Tiwari, R., Chakraborty, S., Dhama, K.: Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. BioMed Research International
2014, 19 (2014). doi:10.1155/2014/761264
17. Serebrovskaya, T.V., Manukhina, E.B., Smith, M.L., Downey, H.F., Mallet, R.T.: Intermittent hypoxia: cause of or therapy for systemic hypertension? Experimental biology and medicine
(Maywood, N.J.) 233(6), 627-650 (2008). doi:10.3181/0710- mr-267
18. Serebrovskaya, T.V., Xi, L.: Intermittent hypoxia training as non-pharmacologic therapy for cardiovascular diseases: Practical analysis on methods and equipment. Experimental
Biology and Medicine 241(15), 1708-1723 (2016). doi:10.1177/1535370216657614
19. Fulco, C.S., Beidleman, B.A., Muza, S.R.: Effectiveness of preacclimatization strategies for high-altitude exposure. Exercise and sport sciences reviews 41(1), 55-63 (2013). doi:10.1097/
JES.0b013e31825eaa33
20. Muza, S.R., Beidleman, B.A., Fulco, C.S.: Altitude preexposure recommendations for inducing acclimatization. High altitude medicine and biology 11(2), 87-92 (2010). doi:10.1089/
ham.2010.1006
21. Navarrete-Opazo, A., Mitchell, G.S.: Therapeutic potential of intermittent hypoxia: a matter of dose. American journal of physiology. Regulatory, integrative and comparative physiology
307(10), R1181-1197 (2014). doi:10.1152/ajpregu.00208.2014
22. Varghese, F., Bukhari, A.B., Malhotra, R., De, A.: IHC Profiler: An Open Source Plugin for the Quantitative Evaluation and Automated Scoring of Immunohistochemistry Images of Human Tissue Samples. PloS one 9(5), e96801 (2014). doi:10.1371/ journal.pone.0096801
23. Edwards, C., Heath, D., Harris, P., Castillo, Y., Kruger, H., Arias-Stella, J.: The carotid body in animals at high altitude. The Journal of pathology 104(4), 231-238 (1971). doi:10.1002/
path.1711040404
24. Prabhakar, N.R., Jacono, F.J.: Cellular and molecular mechanisms associated with carotid body adaptations to chronic hypoxia. High altitude medicine and biology 6(2), 112-120 (2005).
doi:10.1089/ham.2005.6.112
25. Bhatia M, K.T., Trapani GD, and Tonissen KF: The Interaction Between Redox and Hypoxic Signalling Pathways in the Dynamic Oxygen Environment of Cancer Cells. In: Tonissen, K.
(ed.) Carcinogenesis. pp. 125-152. InTech, (2013)
26. Prabhakar, N.R., Semenza, G.L.: Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiological reviews 92(3), 967-1003 (2012). doi:10.1152/physrev.00030.2011
27. Michiels, C.: Physiological and pathological responses to hypoxia. The American journal of pathology 164(6), 1875-1882 (2004). doi:10.1016/s0002-9440(10)63747-9
28. Dvorakova, M., Hohler, B., Vollerthun, R., Fischbach, T., Kummer, W.: Macrophages: a major source of cytochrome b558 in the rat carotid body. Brain research 852(2), 349-354 (2000).
29. Gorlach, A., Dimova, E.Y., Petry, A., Martinez-Ruiz, A., Hernansanz- Agustin, P., Rolo, A.P., Palmeira, C.M., Kietzmann, T.: Reactive oxygen species, nutrition, hypoxia and diseases: Problems solved? Redox biology 6, 372-385 (2015). doi:10.1016/j. redox.2015.08.016
30. Li, C., Jackson, R.M.: Reactive species mechanisms of cellular hypoxia-reoxygenation injury. American journal of physiology. Cell physiology 282(2), C227-241 (2002). doi:10.1152/ajpcell. 00112.2001
31. Acker, T., Fandrey, J., Acker, H.: The good, the bad and the ugly in oxygen-sensing: ROS, cytochromes and prolyl-hydroxylases. Cardiovascular Research 71(2), 195-207 (2006). doi:10.1016/j. cardiores.2006.04.008
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License